What structural units make up ionic solids?
1 Answer
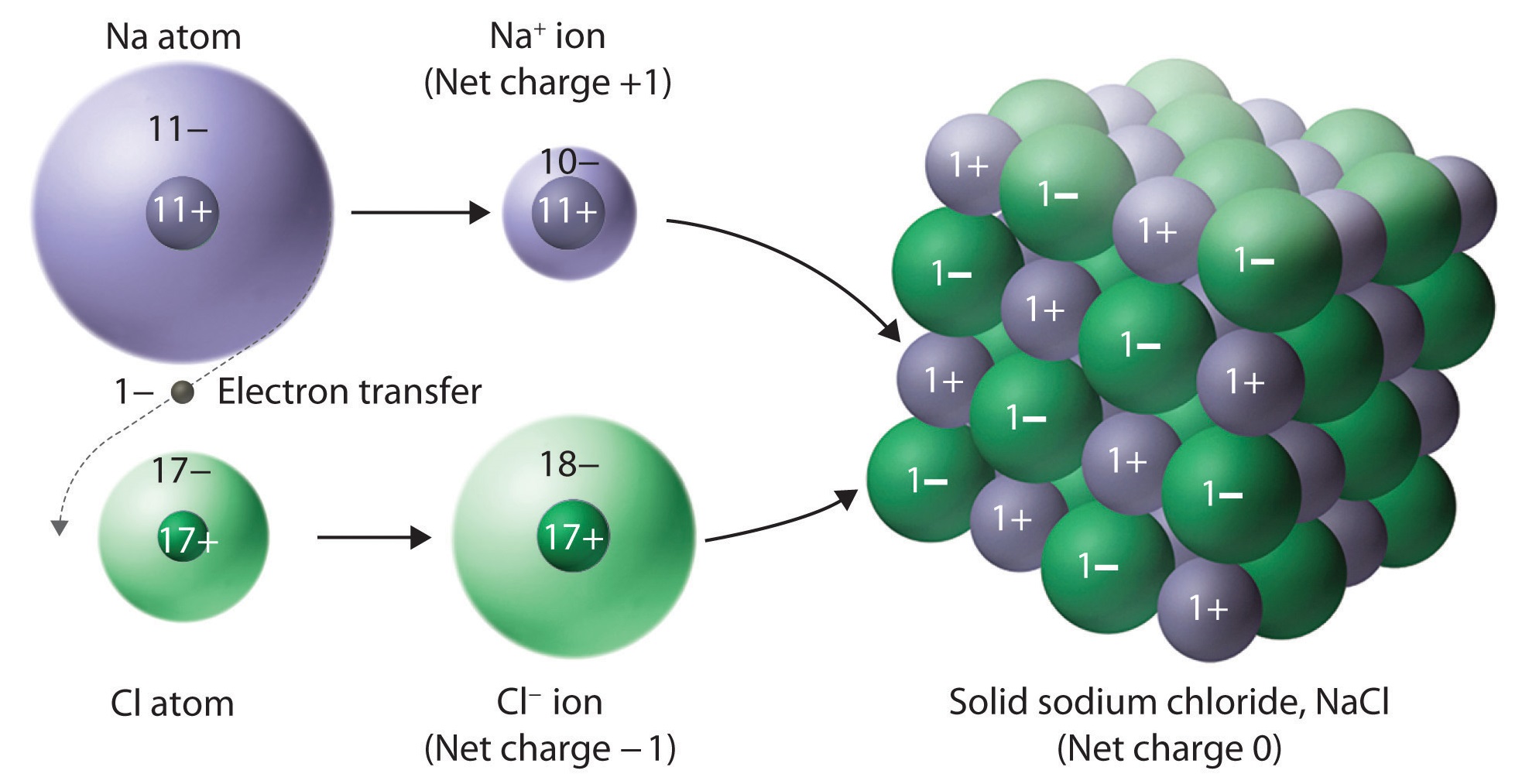
A typical ionic solid consists of a geometric array of ions, alternating between the cation and anion:
 chem.libretexts.org
chem.libretexts.org
It is for this reason why it is generally inappropriate to call a single unit of an ionic compound a "molecule", which usually implies a covalent bond. Instead, an individual unit (one cation, one anion) is called a formula unit (in the image above, it is
The electrostatic attractions between oppositely-charged ions (
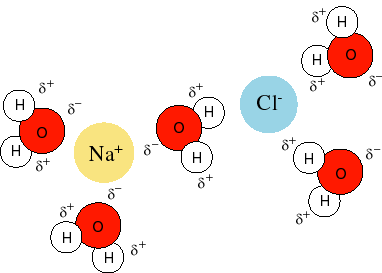
When an ionic compound is placed in water, the ionic compound generally dissociates into its component ions via the polarity of water molecules; the positive end of several water molecules (the
This process is called solvation:
 http://www.public.asu.edu
http://www.public.asu.edu
Notice how the negative end of the water molecule (oxygen end, symbol
- So what structural units comprise an ionic solid?
Possibly the ions themselves!
