What transition metals are radioactive?
1 Answer
Aug 1, 2017
Tc, Lr, Rf, Db, Sg, Bh, Hs, Mt, Ds, Rg, and Cn
Explanation:
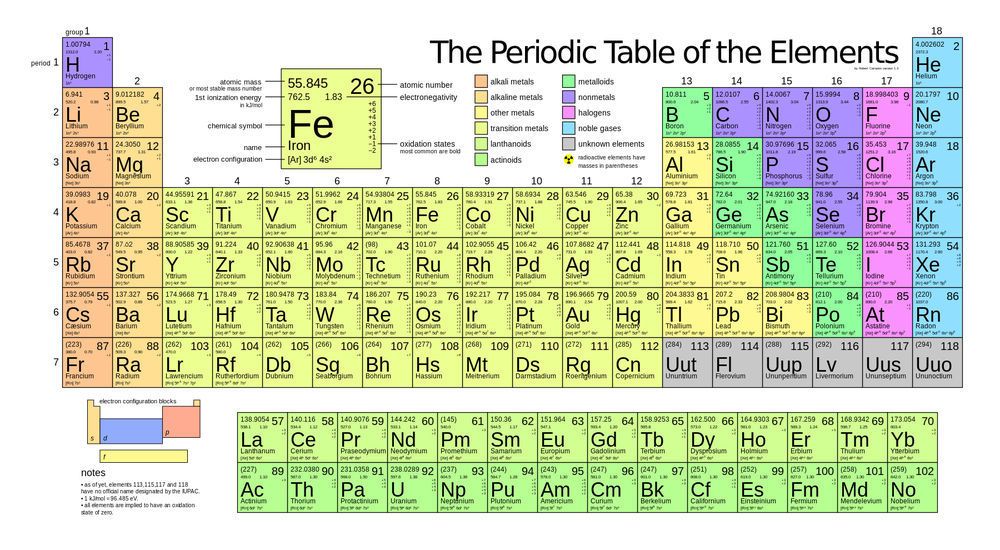
The transition metals are the yellow elements in the middle of the periodic table. When looking at a periodic table, the atomic mass of each element is shown; for example, carbon has an atomic mass of 12.011. When atoms have no known stable isotopes, the atomic number is displayed as a whole number in parenthesis, such as for technetium: (98). While certain isotopes of all elements can be radioactive, an element is considered radioactive if it has no stable isotopes. The transition metals with no stable isotopes are:
Tc, Lr, Rf, Db, Sg, Bh, Hs, Mt, Ds, Rg, and Cn. Notice that all except Tc are transuranic, meaning that their atomic number is greater than 92.

