What is the oxidation number of carbon in the salt sodium oxalate, #Na_2^(+)C_2O_4^(2-)#?
3 Answers
Explanation:
We assign oxidation states to atoms in a molecule by basically using one premise: the more electronegative atom gets all the bonding electrons.
Here we are considering the oxalate ion:
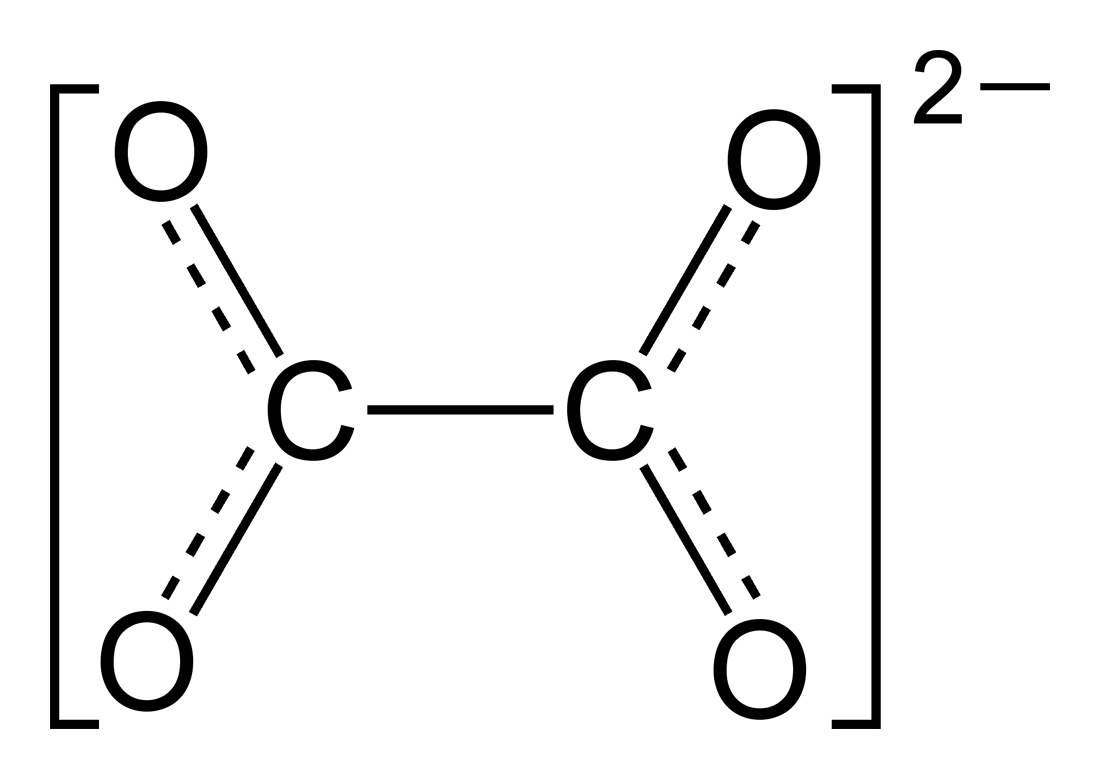
We know that oxygen is more electronegative than carbon and it is typically assigned an oxidation number of
So
As the whole complex ion has a charge of
i.e.
We have
Explanation:
By definition, the oxidation state is the charge left on the central atom, when all the bonding atoms are removed with the charge (the electrons) devolved to the most electronegative atom.....
We gots
And so
And
And
Another way we could look at this is to split the
answer = 3 [oxidation state]
Explanation:
Oxygen is one of the most electronegative elements after flourine so it shows 2 as oxidation state for most compounds [exception - hydrogen peroxide
SO IN
oxidation state of carbon will be positive .
.°. 2(oxidation state of carbon) + 4 ( oxidation state of oxygen) = -2
2 (C) + 4 (-2) = -2
2(C) = 6
OXIDATION STATE OF CARBON IS



