Question #aaac1
1 Answer
Movement of energy with
Explanation:
We all are quite aware of the fact that metals have 'free electrons'.
But generally, we take the availability of free electrons as something which helps in the conduction of electricity due to the availability of free
This helps in the conduction of electricity.
Now, let's relate conduction of heat with conduction of electricity.
 http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/energy/heatrev1.shtml
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/energy/heatrev1.shtml
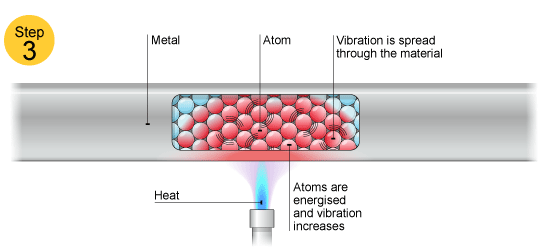
Electrons can move throughout the metal so if the metal is heated (just the end or any small portion) heat will spread all over.
This is because the electrons present take up energy and then when they wander all over the metal, they conduct heat due to their motion.
Upon heating, electrons get excited and this causes their movement or 'conduction of heat'.
Hope it helps!
CHEERS!


