What are examples of heterolytic and homolytic cleavage? Could the former happen with methane?
1 Answer
HETEROLYTIC CLEAVAGE
Heterolytic cleavage is asymmetrical breaking of a bond, giving the electrons to one atom preferentially over the other.
It occurs most often through direct electron flow from another molecule, polarizing the electron cloud of the reactant so that one atom gets more electron density than the other during the bond breaking process.
Here is an example with addition of
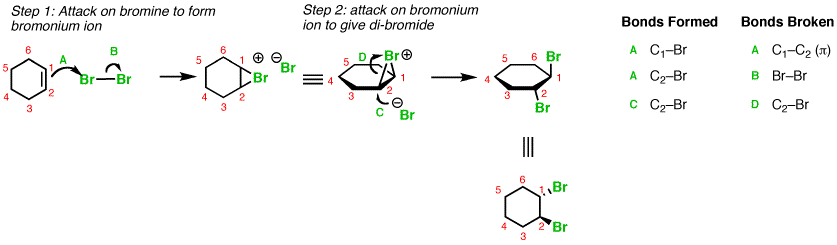
You can see that the alkene polarizes the
Here is an example using a polar reactant:
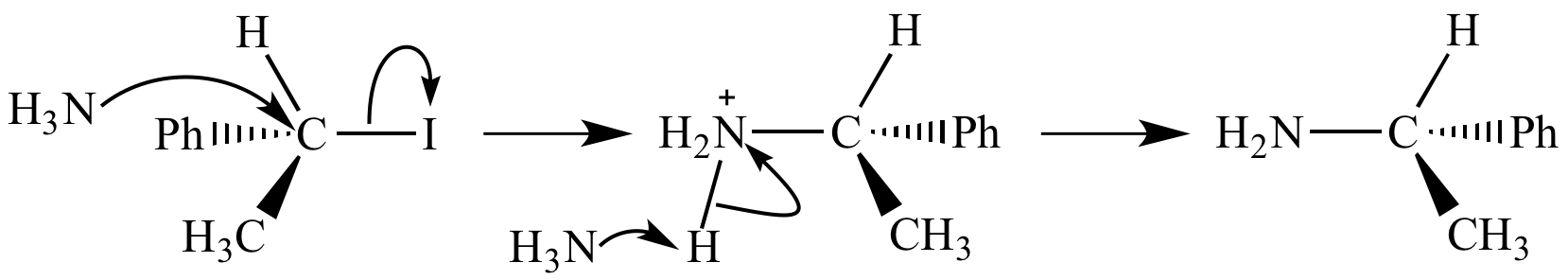
In the first step,
HOMOLYTIC CLEAVAGE
Homolytic cleavage, symmetrical bond breaking, generally occurs in nonpolar molecules, especially ones hit by UV light (
These typically happen in radical reactions, and if one radical is already present, homolytic cleavage becomes more likely.
-
Initiation
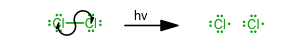
(Without this step, the reaction cannot proceed.) -
Propagation
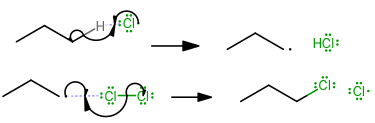
(Here, the reactants form the necessary intermediates to form the product.) -
Termination
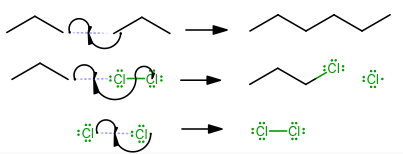
(Here, the reactants react such that they no longer become reactive, hence finishing the reaction.)

