Why does oleic acid have a higher viscosity than water?
1 Answer
Oleic acid has a higher viscosity than water because it is a much larger molecule than water with larger dispersion forces.
Explanation:
Oleic acid is a chain of 18 carbon atoms. Seventeen of the carbon atoms are in a long hydrocarbon chain.

(a) London dispersion forces
The most important intermolecular force in hydrocarbon chains consists of London dispersion forces.

The longer the chain, the greater the accumulated London dispersion forces, so oleic acid is more viscous than water.
(b) Chain entanglement
Although we call oleic acid a "straight-chain" molecule, it is a very "floppy" molecule because there is free rotation about almost every carbon-carbon bond.
This "floppiness" means that the chains can get entangled with each other.
The longer the chain, the greater the entanglement.
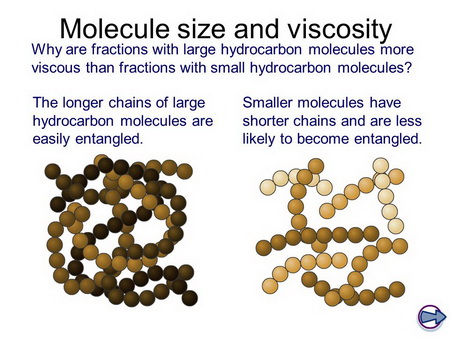
(From SlidePlayer)
As the chains become entangled, it becomes more difficult for the molecules to move past each other, so the viscosity increases.
Chain entanglement may be a more important actor than London dispersion forces in determining the viscosity of oleic acid.

