How would you define reduction in terms of the loss and gain of oxygen?
1 Answer
Aug 7, 2017
reduction is the loss of oxygen
Explanation:
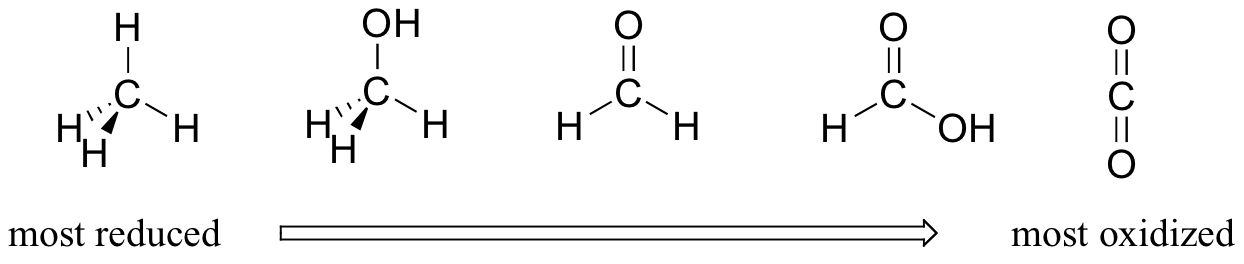
Oxidation is loss of electrons. Reduction is gain of electrons. Since oxygen is a highly electronegative element, most of the electron density would be surrounding the oxygen. Let's consider organic molecules (as in the following picture). When oxygen is added to the molecule, the carbon becomes more oxidized, especially when double bonds are involved. When removing oxygen and replacing with hydrogen, carbon becomes more reduced.