Why does volume of a balloon filled with air increase as its temperature the increases? (Assume constant pressure.)?
1 Answer
Aug 15, 2017
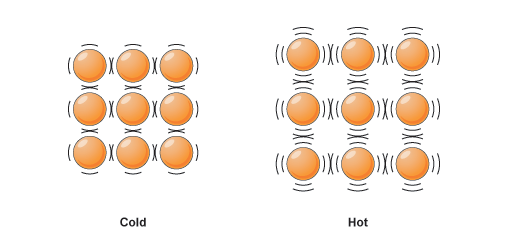
When energy is added, the particles speed up and the volume changes to accommodate that.
Explanation:
A balloon is filled with millions of atoms of air (or whatever fills up the balloon). These particles behave normally, in which when energy is added - heat, the particles take up more volume.
This is generally why when things are hot, they typically expand (at least to some degree).
This is the opposite for cold things, in which their volume decreases.
If pressure was not constant, we would have to consider Gay-Lusaac's Law where:
Hope this helps :)

