Question #10924
1 Answer
Here's what I got.
Explanation:
I'm guessing that you want to figure out a possible set of quantum numbers that can describe an electron located in the
For starters, you should know that we can use a total of
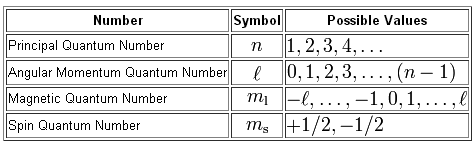
Now, the subshell in which an electron resides is given by the angular momentum quantum number,
#l = 0 -># designates the s subshell#l=1 -># designates the p subshell#l = 2 -># designates the d subshell#l = 3 -># designates the f subshell
#vdots#
and so on. So in your case, all the electrons that can reside in the
#l = 3#
Notice that the value of the angular momentum quantum number depends on the value of the principal quantum number,
This means that in order for your electrons to have access to the
#n = color(red)(5)#
The
#m_l = {-3, - 2, -1, 0, 1, 2, 3}#
The spin quantum number,
#m_s = { -1/2, + 1/2}#
Since each orbital can hold a maximum of
#7 color(red)(cancel(color(black)("orbitals"))) * "2 e"^(-)/(1color(red)(cancel(color(black)("orbital")))) = "14 e"^(-)#
This implies that you can write a total of
For example, you can have
#n =5 , l= 3 , m_l = -3, m_s = +1/2# #n = 5, l = 3, m_l = 0, m_s = -1/2# #n = 5, l = 3, m_l = 2, m_s = +1/2# #n = 5, l = 3, m_l = -1, m_s = -1/2#
and so on. Notice that all the

