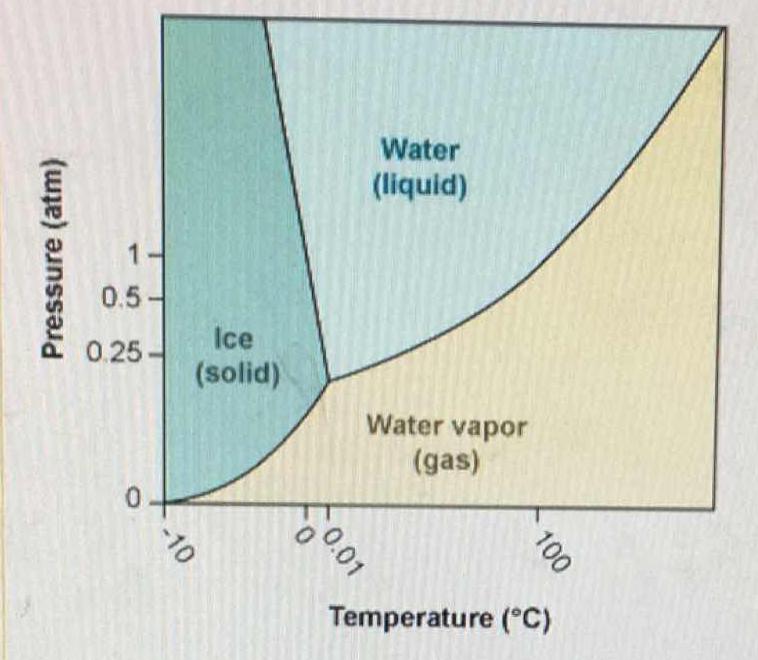
Using the phase diagram for #H_2O#, how would you describe water at 0°C and 1 atm?
1 Answer
At this pressure and temperature water could exist as a liquid, as a solid, or as both.
Explanation:
So what does this mean?
The freezing temperature of water is also its melting temperature. At a temperature of
If you have ice at this temperature and pressure and add heat to the ice it will melt.
Liquid water at this temperature will freeze it heat is removed from the water.
The same events would also occur if you have a mixture of some liquid water and some solid ice. Adding heat will cause an increase in melting. Removing heat will cause an increase in freezing.
Note: The temperature of the water will only go below the freezing point after all of the water has turned into ice. Likewise, the temperature can only be raised above
This video provides further discussion of phase changes for water and takes a closer look at phase diagrams.
Hope this helps!

