X-rays are formed when electrons that have been accelerated to high speeds collide with a target metal. Typical voltages used to accelerate these electrons range from #10,000V- 200,000V#. Due to the acceleration caused by this high potential difference, the electrons gain a high kinetic energy. When they strike a the target metal or anode this energy is converted mainly to heat in the metal target, but some of the energy is emitted as very high frequency(hence low wavelength).
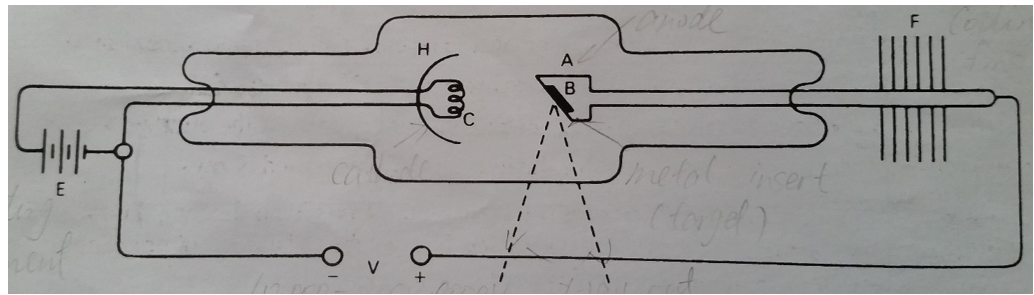
#A# high potential difference #V# is applied between the cathode #C#and the target anode #A#. #E# is a power source that drives a current through the cathode and this heats the cathode. As the cathode is heated this provides energy to the electrons in the cathode, thus enabling them to leave the cathode more easily. Under the influence of the high potential difference #V(10,000 - 200,000)#, the electrons are ripped out of the cathode and accelerated to the anode #A#. Then electrons collide with the atoms and nuclei of anode #A#, that is when x-rays are formed.

