What does a positive or negative liquid-solid slope indicate in a phase diagram?
1 Answer
Aug 27, 2017
When you already know the
Consider the phase diagram of water.
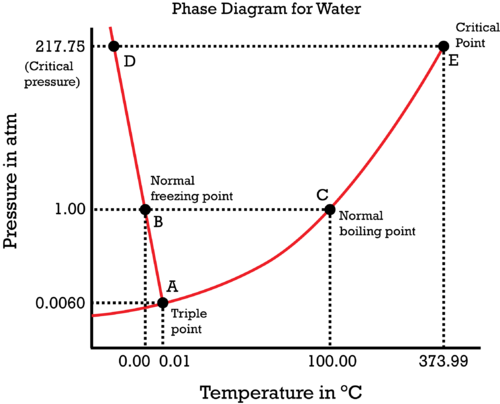
The liquid-solid coexistence curve is
#(dP)/(dT) = (DeltabarH)/(TDeltabarV)#
Consider
- We know that the enthalpy of fusion for melting water is
#DeltabarH_"fus" = "6.02 kJ/mol"# , or#"60.20 L"cdot"bar/mol"# , a positive quantity. - We also know that the temperature
#T# in#"K"# must be positive.
Since the slope of
#(-) = ((+))/((+)(?))#
Thus, the change in molar volume
In other words, ice contracts when it melts and water expands when it freezes.

