Explain the difference in the solubility of water and hydrogen sulfide in hexane? Solubility of water in hexane is 0.01 g/100g Solubility of hydrogen sulfide in hexane is 0.7g/100g?
1 Answer
Here's my take on this.
Explanation:
The key here is the fact that hydrogen sulfide has a smaller dipole moment than water.
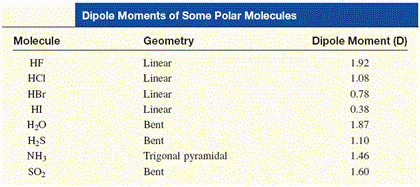
This means that hydrogen sulfide is not as polar as water. Since hexane is a nonpolar molecule, the solubility of a molecule in hexane will depend on whether or not that molecule is polar or nonpolar and, consequently, on how polar that molecule is.
As you know, like dissolves like, so right from the start, the fact that both water and hydrogen sulfide are polar molecules should explain their relatively low solubility in hexane.
The difference between the solubility of water and the solubility of hydrogen sulfide can be attributed to the fact that water is more polar than hydrogen sulfide.
The bigger dipole moment of water means a greater separation of charge between the partial-positive end of the molecule and the partial negative end of the molecule.
By comparison, the smaller dipole moment of a hydrogen sulfide means a less significant separation of charge between the partial-positive end of the molecule and the partial negative end of the molecule
Consequently, hydrogen sulfide will be more soluble in hexane compared with water.

