How do atoms combine?
1 Answer
Sep 11, 2017
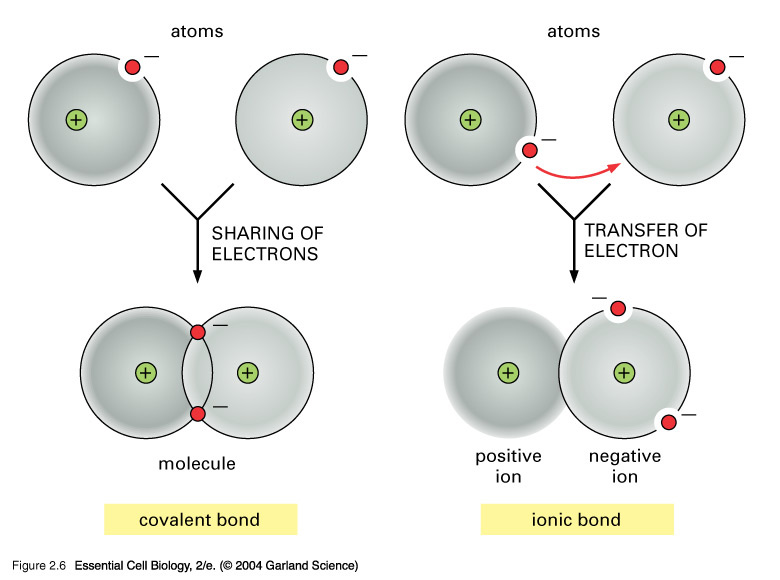
By the sharing of electron density, or by the exchange of electrons to form discrete positive and negative ions.....
Explanation:
The modern covalent bond is conceived to be a region of high electron density between 2 positively charged atomic nuclei such that internuclear repulsion is negated, and a net attractive force operates between the nuclei and the electron cloud.
Alternatively, atoms can exchange electrons to form discrete positive and negative ions, which may be bound together in a non-molecular, electrostatic lattice.