How does polarity affect ionic bonds?
1 Answer
See below.
Explanation:
Here polarity is such type of matter when a ionic compound gets covalent property.
We can understand this property by 'Fajan's Rule'.
For polarisation the effect to a ionic compound are that---
1)The boiling point of the compound decreases.
2)The melting point of the compound decreases.
3)The compound's solubility in polar solvent decreases but in nonpolar solvent increases.
etc.
All of this because the ionic compound becomes more polar.
When the cation is too small and the anion is too big,the cation strongly attracts the electron and the electrons present in the outermost shell of the anion gets attracted by the cation so the normal size of the anion changes and its size is distorted.
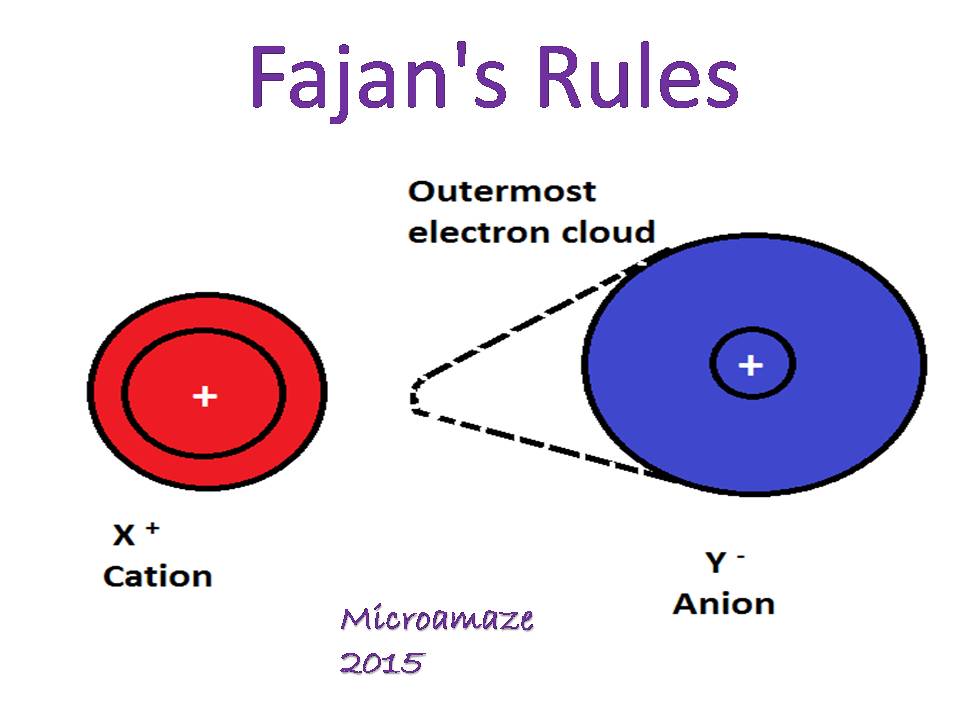
The dotted line shows how the size of the anion changes.
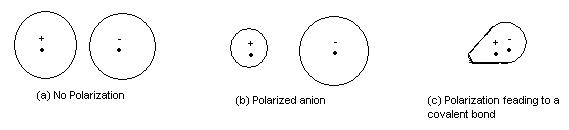
Here it is shown best.
Hope it helps...
Thank you :)

