Question #d6555
2 Answers
I would heat the system:
Explanation:
I am thinking of a gas, at a certain temperature, in a cylinder with a piston. When we free the piston the gas will expand, pushing the piston, and getting colder.
We can heat the cylinder comunicating energy to the gas and compensating the cooling maintaining the temperature constant.
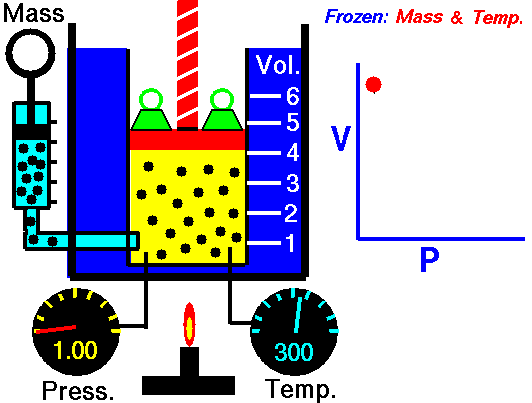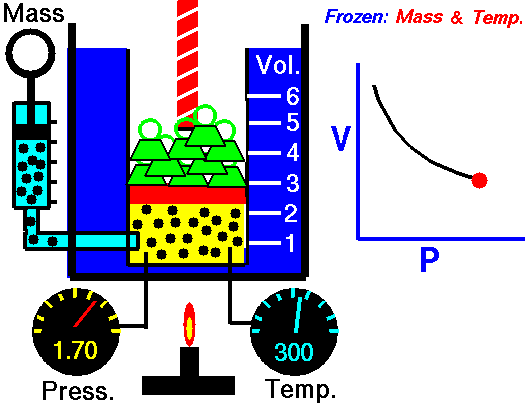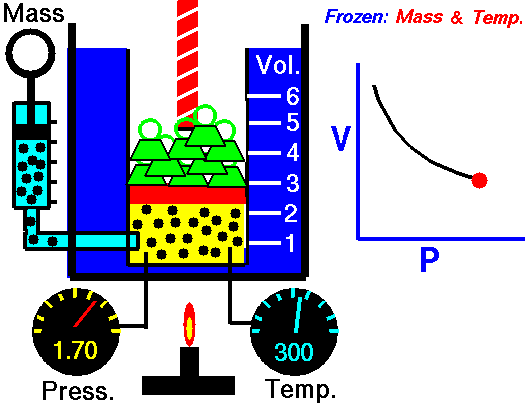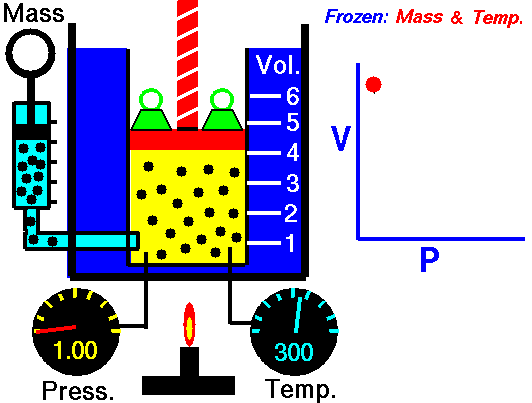
Decrease the pressure.
Explanation:
Lets start with the facts. There are 3 variables for gases, temperature, volume and pressure. In your example temperature is a constant, that leaves volume and pressure as the variables. If the gas is allowed to expand then the volume is increasing.
Boyle's law states that pressure and volume are inversely proportional. If the volume is increasing then the pressure needs to decrease in order to keep the temperature the same.
If you can imagine a pressure cooker, as heat is being added your pressure increases because volume stays the same, and the temperature of the gas increases. The only way to allow the gas to expand is to release the pressure. With the pressure valve released the gas is free to expand and the temperature can remain constant.