Question #92aa1
1 Answer
Explanation:
Going through it logically, we first have to understand what density means, density is mass over volume as in definition, but to understand it, density is how many thing you have in a certain area or space.
Let us take the example here:
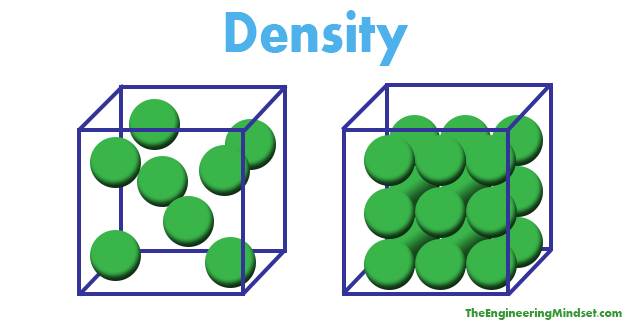
Let us say that each box is 1
If you look the one on the right, we have 8 balls in the box, so its density is 8balls per
While the left box has 22 balls per
Her we can see that the higher the density, the more we have at the same volume.
In your example, gold has higher density than silver.
Going through it mathematically
The question wants to find which has greater mass 5
Using the density definition:
Multiplying both sides by volume to get the formula for mass:
Mass of gold:
Mass of silver:

