The quantum numbers n = 4, l = 1, m_l = 0 could represent a valence electron in which atom in its ground state?
Answer choices are Fe, In, Pd, and Se, and answer key says it is Se.
I know that these quantum numbers refer to 4s electrons, so I eliminated B and C. How can I tell if the answer is Fe or Se, since both seem to have 4s electrons?
Thanks!
Answer choices are Fe, In, Pd, and Se, and answer key says it is Se.
I know that these quantum numbers refer to 4s electrons, so I eliminated B and C. How can I tell if the answer is Fe or Se, since both seem to have 4s electrons?
Thanks!
1 Answer
Nov 14, 2017
Explanation:
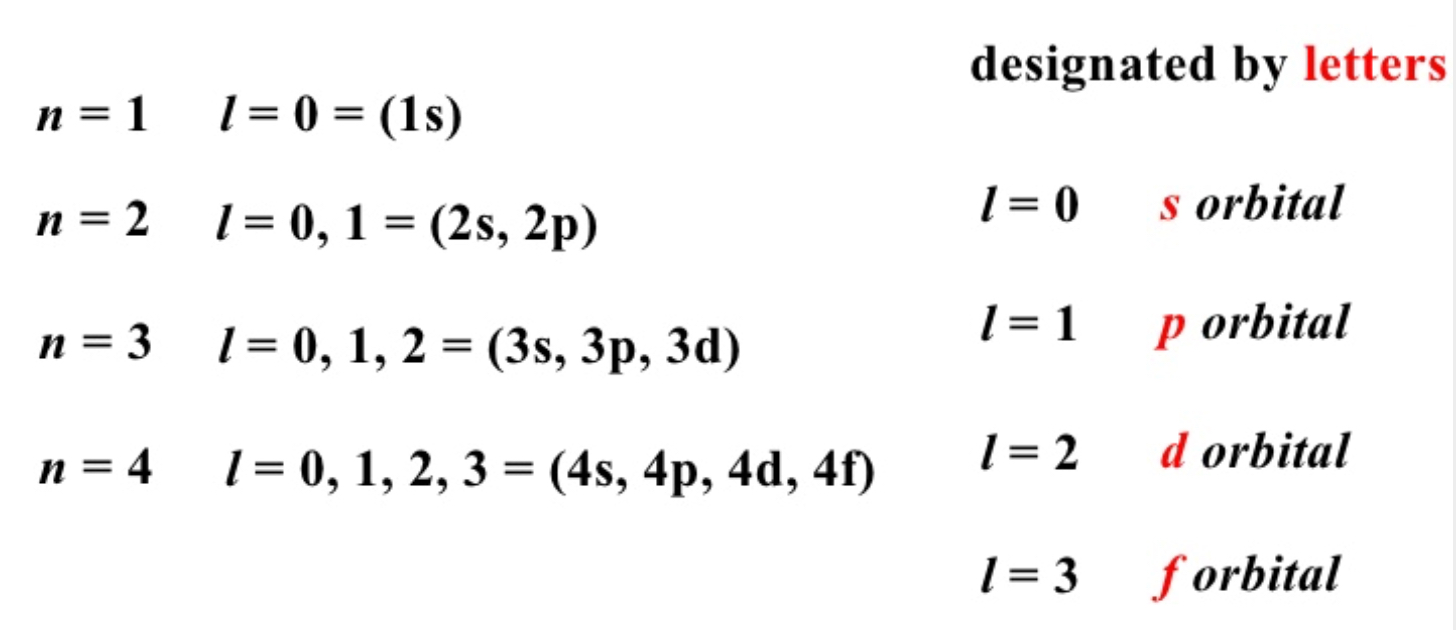
In p-block elements, the last (valence) electron enters in the outermost p-orbital. Therefore, atom belongs to p-block.
- Fe is d-block element
- Se is p-block element
Se is the correct answer.

