Boric acid can take only one electron pair due to absence of d orbital.What does this means?
1 Answer
The valence shell of a boron atom is the second shell, which does not include
Explanation:
Remember that the electron configuration of boron is
The boron atom at the centre of the boric acid molecule (see the diagram) is covalently bonded to each of three oxygen atoms, using
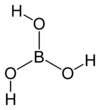
This leaves one empty orbital in the valence shell of the boron atom, and makes it possible for the molecule to gain an electron pair by forming a "coordinate covalent bond" with another particle that has a lone pair (a full, unbonded orbital) in its valence shell.
However, because the boron atom's valence shell is the second shell, and consists of only
Additional

