Compare the structures of SO3 to PF3 and explain why they have different molecular shapes?
(when comparing two objects, you should discuss both objects, you should discuss both and mention both their similarities and differences and explain the reason for the differences)
(when comparing two objects, you should discuss both objects, you should discuss both and mention both their similarities and differences and explain the reason for the differences)
1 Answer
See below.
Explanation:
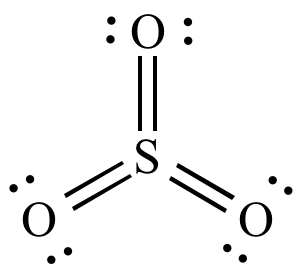 http://www.chem.ucla.edu/~harding/IGOC/S/sulfur_trioxide.html
http://www.chem.ucla.edu/~harding/IGOC/S/sulfur_trioxide.html
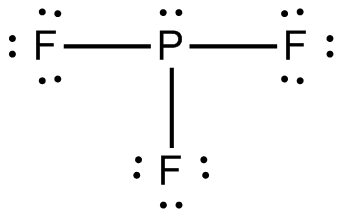 http://pixshark.com/pf3-lewis-structure.htm
http://pixshark.com/pf3-lewis-structure.htm
Similarities:
-
Both are bonded to 3 atoms.
-
Both don't have any resonance structures (no double bonds in
PF_3 and nowhere else to put the double bonds inSO_3 )
Differences:
-
The central atom in
PF_3 has 1 lone pair of electrons, while the central atom inSO_3 has no lone pairs. -
SO_3 violates the octet rule, whilePF_3 obeys it (3 single bonds around P and 1 lone pair = 8 total electrons, while 3 double bonds around S is 12 total electrons). -
PF_3 has a trigonal pyramidal molecular geometry, compared toSO_3 having a trigonal planar molecular geometry (P is bonded to three atoms and has 1 lone pair, while S is bonded to 3 atoms and has no lone pairs) (see picture below). -
PF_3 will be a polar molecule andSO_3 will be a nonpolar molecule (because the structure ofPF_3 is not symmetrical andSO_3 is symmetrical, meaning the individual dipole moments of each bond will be 'canceled out' inSO_3 , but not inPF_3 , leaving a net dipole moment (polarity) inPF_3.
 http://hootcampapchemistry.wikispaces.com/Bonding?responseToken=76cad330384c2794b05fa609eea382fe
http://hootcampapchemistry.wikispaces.com/Bonding?responseToken=76cad330384c2794b05fa609eea382fe
I hope that's enough and that this helped!

