Question #39c9b
1 Answer
Dec 10, 2017
Group 1
Explanation:
If by K you mean potassium, then it is in group 1.
Potassium is one of the alkali metals, so is in group 1. It has 1 outer shell electron, and starts the new shell. It has an electronic configuration of
You can tell an element's group from its position in the periodic table. The groups are the PT's columns; they are numbered from 1 to 8, (or 1-18 (although sometimes the last group is also called group 0)).
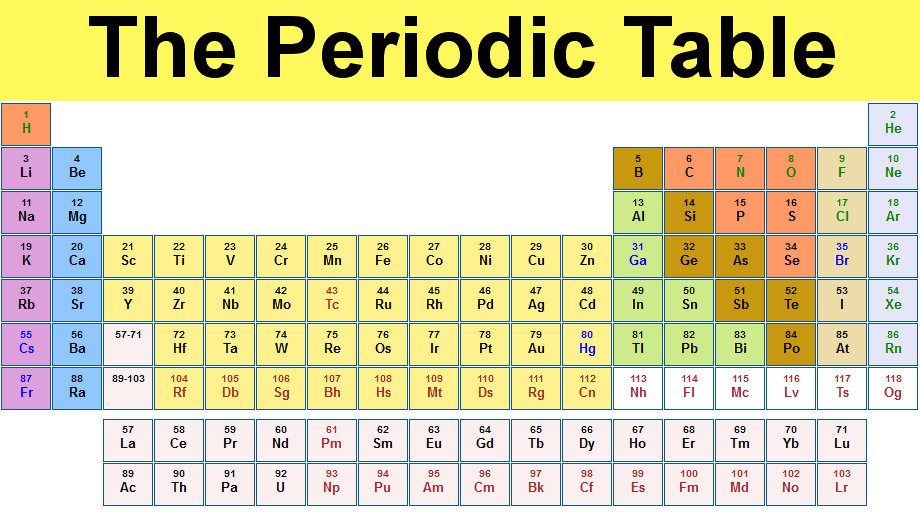
To determine an element's group, count along the columns from the left. So potassium (K) is in the first row from the left, so it is group 1. Carbon (C) is in the forth row from the left, since we skip over the transition metals, so is in group 4. Neon (Ne) is in group 8.
