How to draw a BN molecular orbital diagram?
1 Answer
This is the general MO diagram you need to fill with the valence electrons of BN
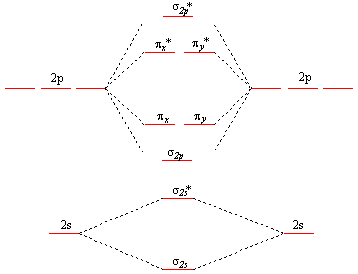
Boron has 3 valence electrons, and nitrogen has 5 valence electrons, this makes 8 electrons.
You have to start filling the orbitals from those with lowest energy to those with higher energy.
So, 2 electrons on σ2s
, two electrons on σ∗2s, two electrons on σ2p
. You have now 2 electrons left, and two orbitals of the same energy.
In this case, you need to follow Hund's rule, which states that if two or more orbitals of equal energy are available, electrons will occupy them singly before filling them in pairs. So you will have to put one electron on the πx
orbital and another one on the πy orbital. So you end up with 2 unpaired electrons, and paramagnetism of the molecule is explained.

