Question #e086c
1 Answer
No, helium is not the only product of the reaction.
Explanation:
The idea here is that you need to write out the unbalanced nuclear reaction that describes this fusion reaction and use the fact that charge and mass are conserved to balance it.
The two isotopes of hydrogen that fuse to produce helium are deuterium, or hydrogen-2, and tritium, or hydrogen-3. These two nuclides fuse to produce helium-4, also known as an alpha particle.
So, you can say that you have
#""_1^2"H" + ""_1^3"H" -> ""_2^4"He" + ""_Z^A?#
Now, in order for mass to be conserved, you need to have--look at the mass numbers of the nuclides!
#2 + 3 = 4 + A#
In order for charge to be conserved, you need to have--look at the atomic numbers of the nuclides!
#1 + 1 = 2 + Z#
Solve these two equations to get
#{( A = 2+ 3 - 4 = 1), (Z = 1 + 1 - 2 = 0) :}#
This means that the unknown particle is a neutron, an elementary particle that has a mass number equal to
#""_1^2"H" + ""_1^3"H" -> ""_2^4"He" + ""_0^1"n"#
You can complete the nuclear equation by adding in energy as a product.
#""_1^2"H" + ""_1^3"H" -> ""_2^4"He" + ""_0^1"n" + color(red)("energy")#
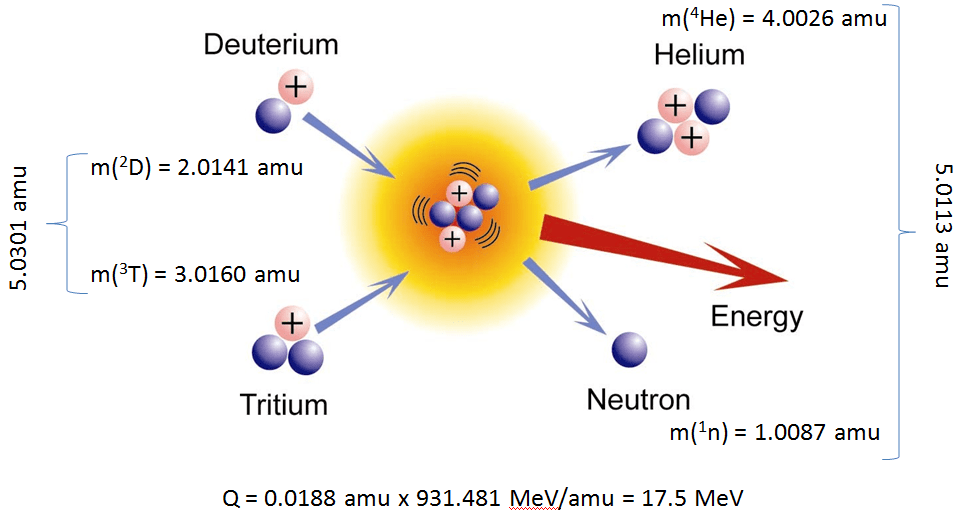
As a side note, it's worth mentioning that the total mass of the reactants is actually smaller than the total mass of the products because some of the mass of the reactants has been converted to energy.
This is perfectly in line with Einstein's concept of Mass-energy equivalence

