How do rationalize atomic size on the basis of an element's position on the Periodic Table?
1 Answer
Dec 21, 2017
Atomic size DECREASES across a Period....
Explanation:
Atomic size DECREASES across a Period, a row of the Periodic Table, from LEFT to RIGHT as we face the Table. It INCREASES down a Group.
This is one of the most important manifestations of atomic structure; i.e. the diminution in size across the Period. Atomic size is a function of (i) nuclear charge, thus higher
Taken together, these two principles rationalize the actual data...
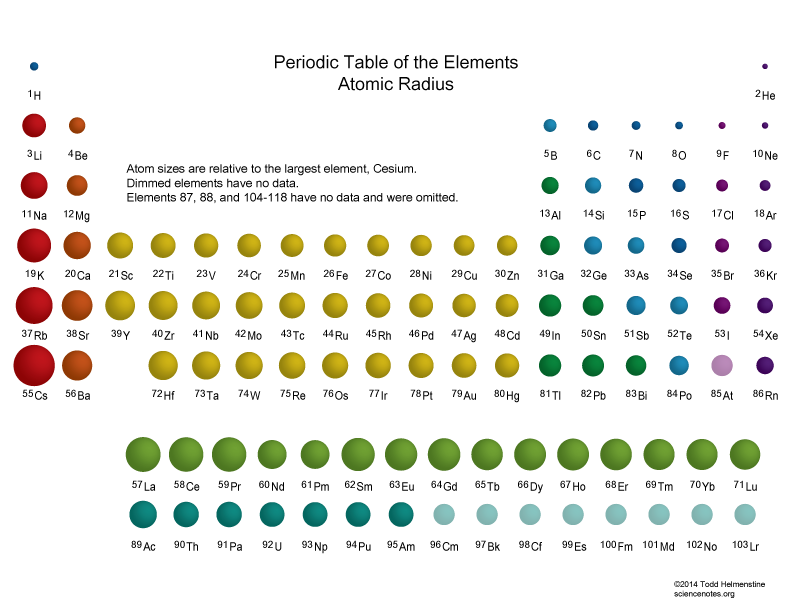
Especially, for the first few short Periods, do these data support what we have argued? And so for a given Period, which is the largest atom?

