What is the balanced chemical equation for the reaction that takes place between bromine and sodium iodide?
1 Answer
Dec 28, 2017
This is a single replacement (single displacement) reaction involving the halogens (group 17/VIIA on the periodic table), rather than metals.
Explanation:
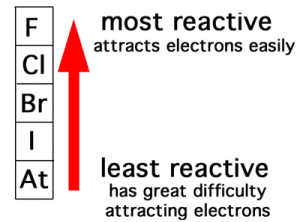
The activity series for the halogens follows the order of the halogens from top to bottom down the group on the periodic table, with fluorine the most reactive halogen and astatine the least reactive. Each halogen can replace the halogen below it in the activity series (or the periodic table).