Equal volumes of #"0.25 M"# #"HNO"_2# and #"0.25 M"# #"HNO"_3# are titrated separately with #"0.25 M"# #"KOH"#. Which would be the same for both titrations?
a) initial pH
b) pH halfway to the equivalence point
c) pH at the equivalence point
d) pH when 5 mL excess KOH has been added
I know the answer is D but I don't understand why, could someone explain? Thanks a million ! (:
a) initial pH
b) pH halfway to the equivalence point
c) pH at the equivalence point
d) pH when 5 mL excess KOH has been added
I know the answer is D but I don't understand why, could someone explain? Thanks a million ! (:
1 Answer
The answer is indeed (d).
Explanation:
!! LONG ANSWER !!
As you know, you're dealing with nitrous acid,
Right from the start, this tells you that point (a) cannot be true because the weak acid does not dissociate completely in aqueous solution to produce hydronium cations.
This implies that the concentration of hydronium cations will be greater in the solution that contains the strong acid, which, in turn, means that the
So in terms of initial
#"pH"_ ("0 HNO"_ 2) > "pH"_ ("0 HNO"_3)#
Now, point (b) cannot be true because, at the half-equivalence point, the
This is the case because the half-equivalence point, the nitrous acid solution will contain equal concentrations of nitrous acid and nitrite anions,
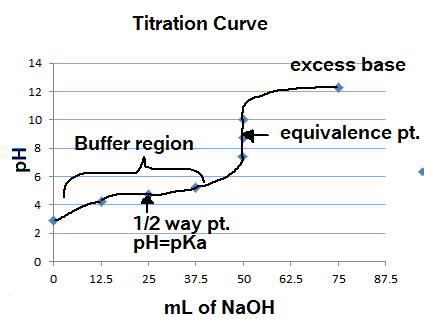
By comparison, at the half-equivalence point, the
This implies that adding the strong base will only reduce the concentration of the hydronium cations produced by the full ionization of the strong acid.
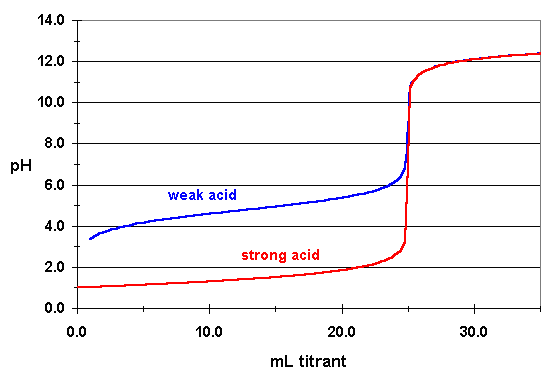
Keep in mind that unlike the nitrite anion, which acts as a weak base, you can assume that the nitrate anion does not act as a base in aqueous solution, i.e. that it will not react with water to reform nitric acid.
Point (c) cannot be true because at the equivalence point, the
This is the case because once the neutralization is complete, the weak acid solution will still contain nitrite anions,
#"NO"_ (2(aq))^(-) + "H"_ 2"O"_ ((l)) rightleftharpoons "HNO"_ (2(aq)) + "OH"_ ((aq))^(-)#
This will cause the
Since the nitrate anion does not react with water to produce hydroxide anions and reform some of the nitric acid, the resulting solution will be neutral and it will have
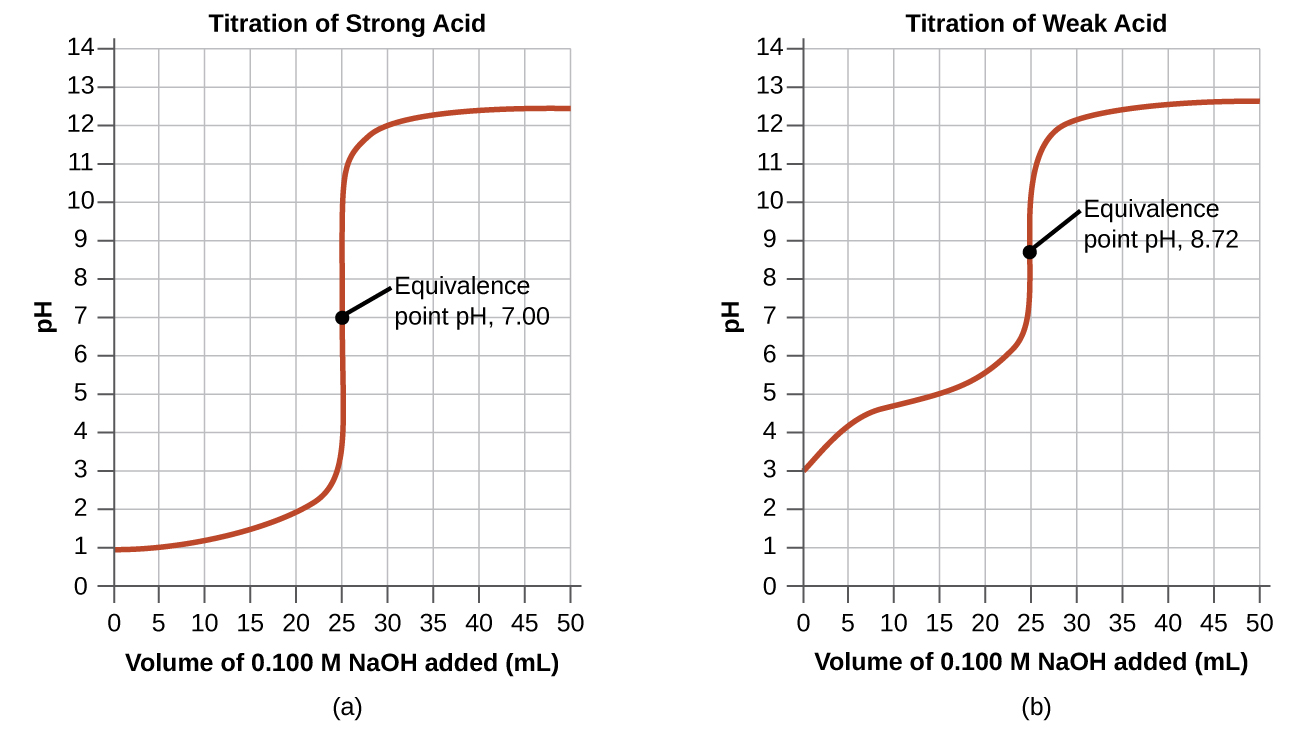
Finally, point (d) is correct because adding
This happens because even though the
In other words, for the weak acid solution, you can use the approximation
#["OH"^(-)] = ["OH"^(-)]_ "already present" + ["OH"^(-)]_ "from excess strong base"#
#["OH"^(-)] ~~ ["OH"^(-)]_ "from excees strong base"#
By comparison, once you add the excess strong base, the strong acid solution, which at the equivalence point contains the very small concentration of hydroxide anions produced by the auto-ionization of water
#["OH"^(-)] = 1 * 10^(-7)color(white)(.)"M" -># a neutral aqueous solution at#25^@"C"#
will have
#["OH"^(-)] = 1 * 10^(-7)color(white)(.)"M" + ["OH"^(-)]_ "from excess strong base"#
#["OH"^(-)] ~~ ["OH"^(-)]_ "from excess strong base"#
This is why the
#"pH" = 14 - [-log(["OH"^(-)]_ "from excess strong base")]#
hence why point (d) is true.

