How many unpaired electrons are in a gaseous Co 3+ ion in its ground state? The answer is 4, but I don’t understand how. Could someone please explain? Thank you!
1 Answer
Jan 1, 2018
A gaseous
Explanation:
The electron configuration of a cobalt atom is
The orbital diagram looks like this:
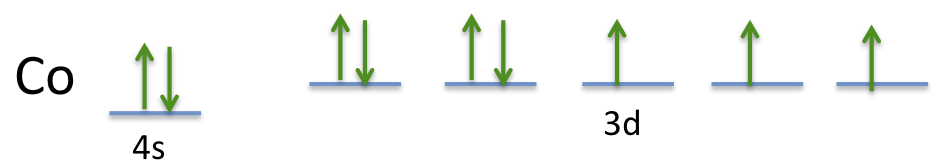
(Adapted from galleryhip.com)
When we remove electrons to form the
The new electron configuration becomes
The new orbital diagram looks like this:
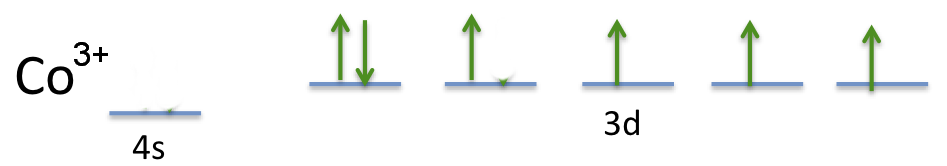
(Adapted from galleryhip.com)
The

