What is asymmetric epoxidation?
2 Answers
All it means is that you are epoxidizing an asymmetric alkene with, for instance, a peroxyacid (this does not work with alkynes due to stability issues of the intermediate), as you have done early in your first semester of organic chemistry.
The concerted mechanism for the peroxyacid epoxidation of an asymmetric alkene is no different from that of a symmetric alkene other than the fact that the alkene looks different.
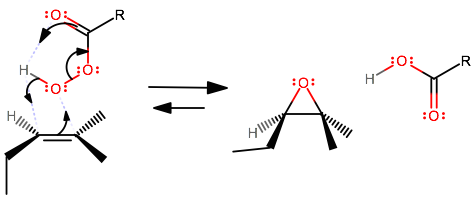
This epoxidation adds in a syn addition, and the enantiomers of this, where the epoxide oxygen is either in the back or in the front of the plane of the molecule, are both formed.
The syn addition is why the mechanism is the same for both symmetric and asymmetric alkenes.
Asymmetric epoxidation (also called the Sharpless epoxidation reaction) is a method of preparing chiral epoxides from prochiral allylic alcohols.
Explanation:
It uses a catalyst containing either (+)- or (-)-diethyl tartrate to form the chiral epoxide in greater than 90 % enantiomeric excess.

The oxidizing agent is tert-butyl hydroperoxide.
The catalyst is prepared from titanium tetra(isopropoxide),
It binds the hydroperoxide, the allylic alcohol group, and the chiral tartrate ligand through the oxygen atoms.
A "simplified" Jmol image of the complex (below) shows how an
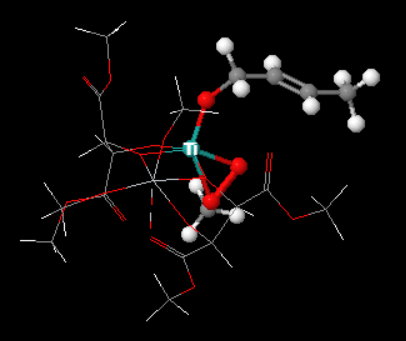
(Adapted from chemtube3d)


