Question #c9ba9
1 Answer
Explanation:
The number of valence electrons can be found by determining the oxidation number.
In the case of
Chlorine is a halogen, which means it needs to gain one electron to achieve noble gas configuration—therefore, its oxidation number in
The oxidation state of all
Because this is a neutral compound (with no charge), the oxidation number of phosphorus has to be
From this, we can infer that phosphorus lost five electrons.
Then, just jump back five spaces on the periodic table:
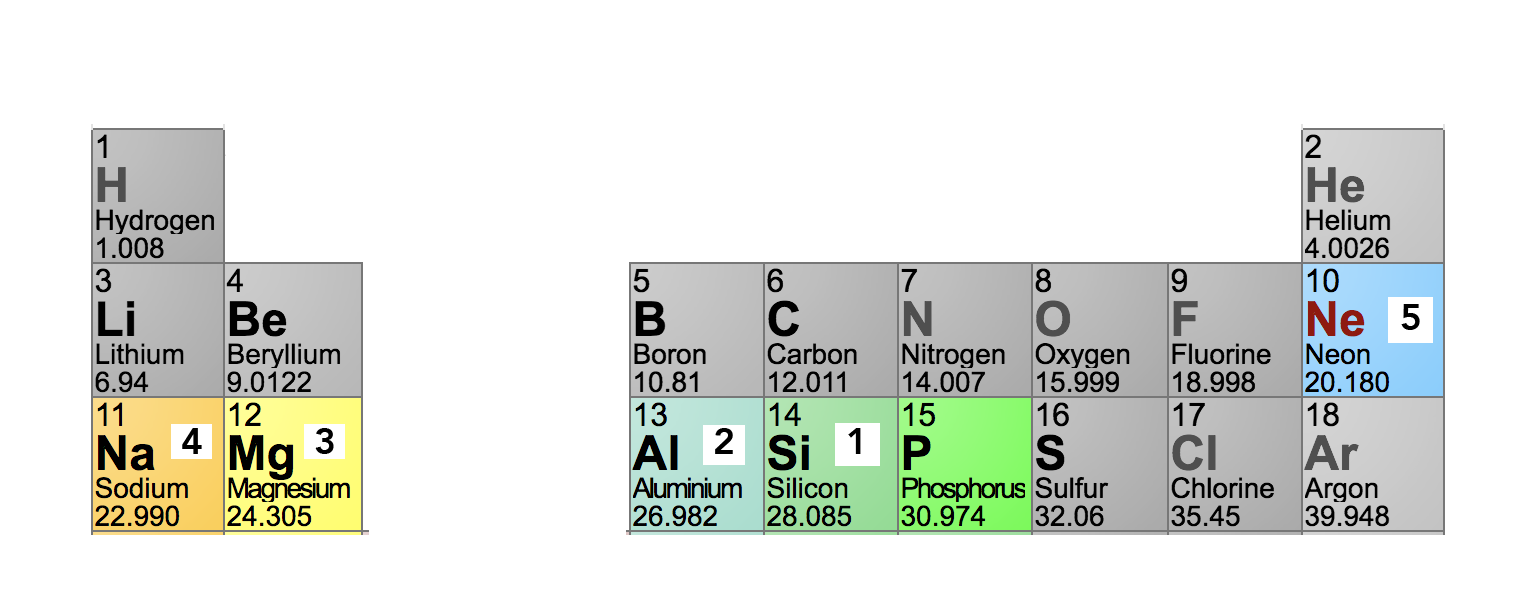
The number of valence electrons
Alternatively, we can look at the Bohr model of phosphorus.
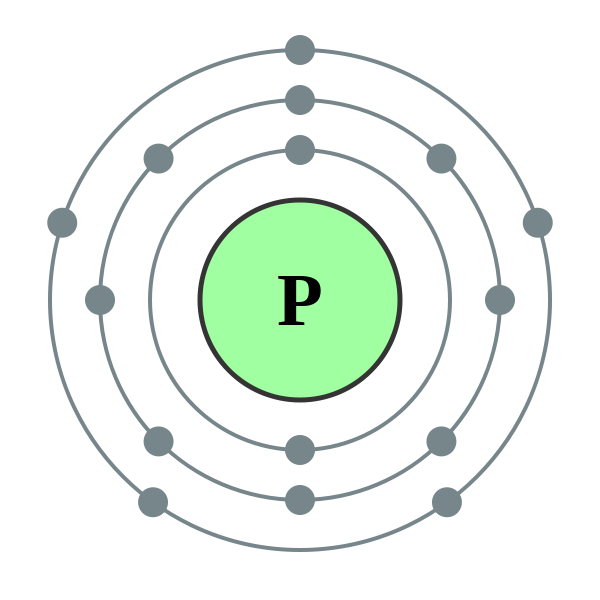
Once all
That shell has

