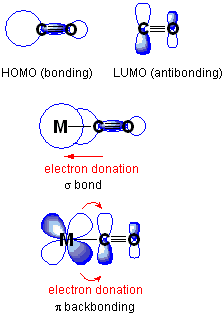
Why do transition metals have variable oxidation states?
1 Answer
Jan 15, 2018
Well, they have many orbitals of similar energy... so they can use them.
The possible oxidation states have already been shown here.
The d-block transition metals often use
...and the f-block transition metals often use
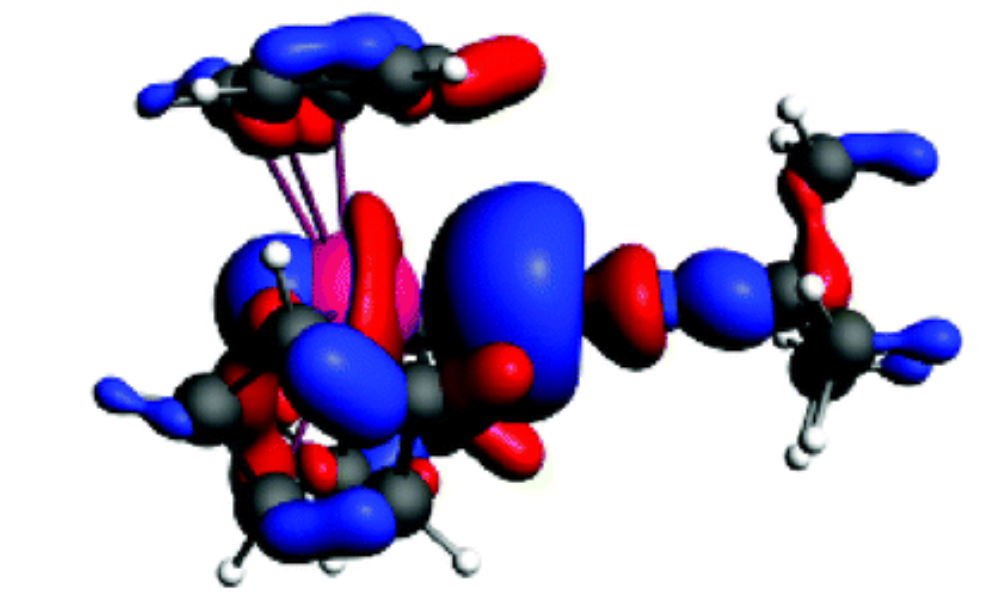
[This is the uranium complex known as tris(cyclopentadienyl)tert-butylisocyanouranium(III).]
Hence, the large pool of valence orbitals allow a flexible selection of oxidation states.