How many moles of each atom are present in a #1.01*g# mass of antifreeze, #C_3H_6O_3#?
2 Answers
Got a Periodic Table...?
Explanation:
And the mole is just a very large number, like a dozen or a gross. For the first problem, we simply calculate the molar mass of sodium chloride.... The Periodic Table tells you that the molar mass of carbon is
The table specifies a
All I am doing is adding up the masses of each mole of atoms, and then taking a quotient. Do you follow?
Explanation:
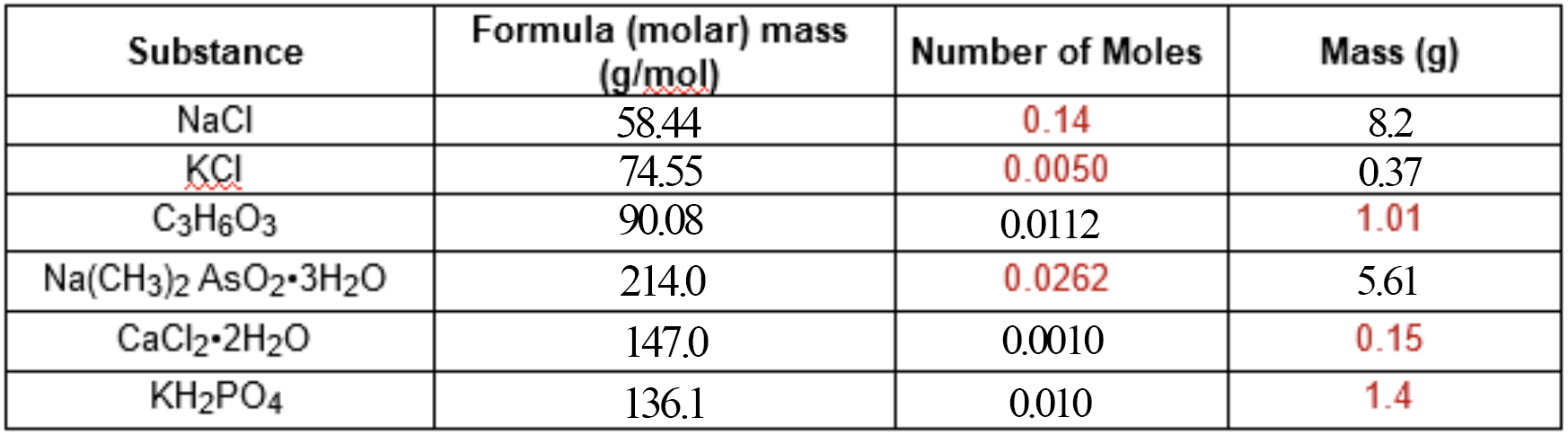
First, let's figure out the molar mass for everything!
Molar mass is just the mass of
-
#NaCl# is#"58.44 g/mol"# , because#Na + Cl = "22.99g + 35.45g" = "58.44g"# -
#KCl# is#"74.55 g/mol"# . -
#C_3H_6O_3# is#"90.08 g/mol"# , because#3*C + 6*H + 3*O = 3*"12.01g" + 6*"1.008g" + 3*"16.00g"= "90.08g"# -
#Na(CH_3)_2AsO_2*3H_2O# is#"214.0 g/mol"# . It looks intimidating, but it's the same principle—we just need to add up the individual element masses! :)
#(Na + 2*(C+3*H) + As + 2*O) + 3*(2*H+O) = (22.99 + 2*(12.01+3*1.008) + 74.92 + 2*16.00) + 3*(2*1.008+16.00)# -
#CaCl_2*2H_2O# is#"147.0 g/mol"# , because, again, we just need to add the molar mass of#CaCl_2# , which is#111.0# , with the mass of#2# moles of water, which is#"36.04g"# -
#KH_2PO_4# is#"136.1 g/mol"# .
Now, let's go over to the second column.
To determine the number of moles from any given mass, just divide the mass of that sample by the mass of
-
#"1.01g"# of#C_3H_6O_3# would be
#1.01/90.08 = "0.0112 moles"# -
#"0.15 g"# of#CaCl_2*2H_2O# would be
#0.15/147.0 = "0.0010 moles"# -
#"1.4 g"# of#KH_2PO_4# would be
#1.4/136.1 = "0.010 moles"#
Let's fill in the second column.
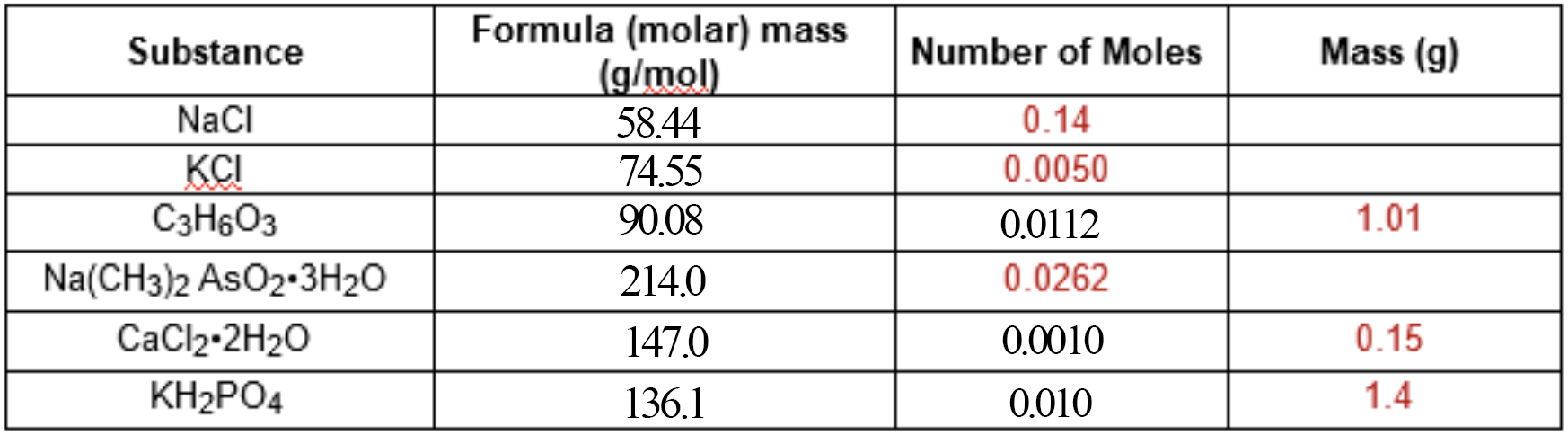
Finally, the third column.
We used this equation for converting from mass to moles:
We can slightly alter this equation to make it suit column
-
#"0.14 moles"# of#NaCl# is
#0.14 * 58.44 = "8.2g"# -
#"0.0050 moles"# of#KCl# is
#0.0050 * 74.55 = "0.37g"# -
#"0.0262 moles"# of#Na(CH_3)_2AsO_2*3H_2O# is
#0.0262 * 214.0 = "5.61g"#



