Why can't ions pass through the lipid bilayer?
1 Answer
Because ions are polar.
Explanation:
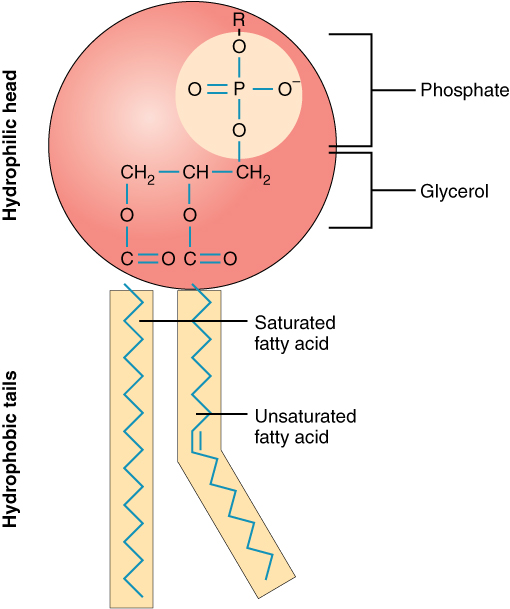
The lipid bilayer layer is actually a phospholipid bilayer made up of a lot of phospholipid molecules. Each phospholipid molecule has following parts:
-
Polar & hydrophilic
#("water-loving")# head :
It is made up of negatively-charged phosphate group#(PO_4^(3-))# and glycerol#(C_3H_8O_3)# molecule. One of the oxygen of phosphate group is attached to a variant, i.e.#"R"# . Thus the nature of phospholipid can vary with the nature of#"R"# . Glycerol is a bridge between the phosphate group and hydrophobic tails. That's why it's known as glycerol backbone. -
Non-polar & hydrophobic
#("water-fearing")# tails : Phospholipid molecule consists of 2 hydrophobic tails made up of fatty acids. The fatty acids chain consist of carbon atoms bonded to hydrogen atoms. One tail is made up of saturated fatty acids, and the other containing double bond is made up of unsaturated fatty acids. Both these tails get attached to the glycerol molecule of the hydrophilic head.
Thus the overall structure of phospholipid is constructed as:
Now consider the rule: Like dissolve like.
So the ions being polar in nature can easily cross the polar and hydrophilic head. But still, they can't enter the cell because their entry gets restricted by the presence of hydrophobic tails. The fatty acid tails being non-polar in nature repel any polar or charged particle and hence don't allow them to enter the cell or escape out of it.
The other factor that contributes is the larger size of ions. The smaller molecules, e.g.
Hope it helps...


