Question #b54c5
2 Answers
Consider a simple amine, ammonia,

Nitrogen in this molecule has a lone pair of electrons that aren't doing anything but increasing its electron density. When a strong acid (e.g.
Since this amine is a nucleophile, it will act as a base, and its electrons will attack the proton from hydrochloric acid, leaving the chloride ion at its (very stable) lonesome.
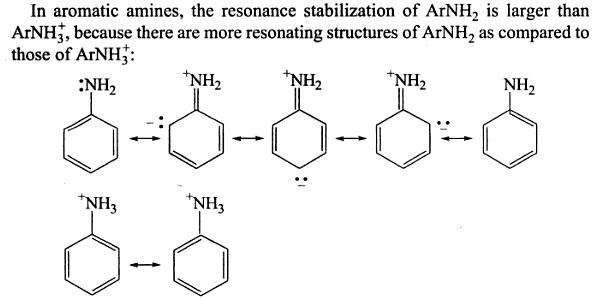
It's important to understand that the electrons in the
See below...
Explanation:
What is Lewis base?
#=># Those compound which can donate lone pairs are called Lewis base.In amine, nitrogen is present which contains lone pair electrons to donate.
These make the amine basic.
There are special cases also.
- When the lone pair electron contribute in resonance,the tendency to loosing electron decreases. Example:- Benzyl amine.