How to calculate maximum beta energy (0.634 MeV) of fluorine-18? I've tried using binding energy formula, but the result is different?!
1 Answer
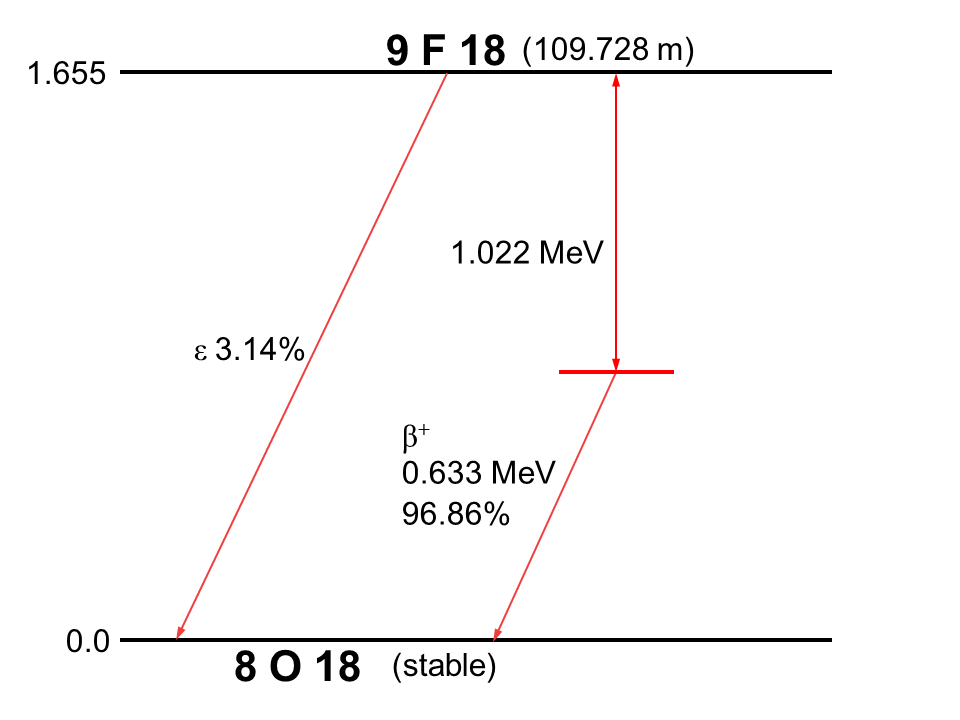
Fluorine-18 decays to Oxygen-18 via positron emission as shown in the figure above. Referring to the part table above we calculate the energy available for the positron emission.
#E=180009380-17.99991610=0.001777\ am u# #=>E=0.001777xx931.5=1.6552755\ Mev#
When a positron is ejected from the parent nucleus, the daughter
Mass of one electron
Now maximum positron energy
Note: For energy difference between parent and daughter nuclei
