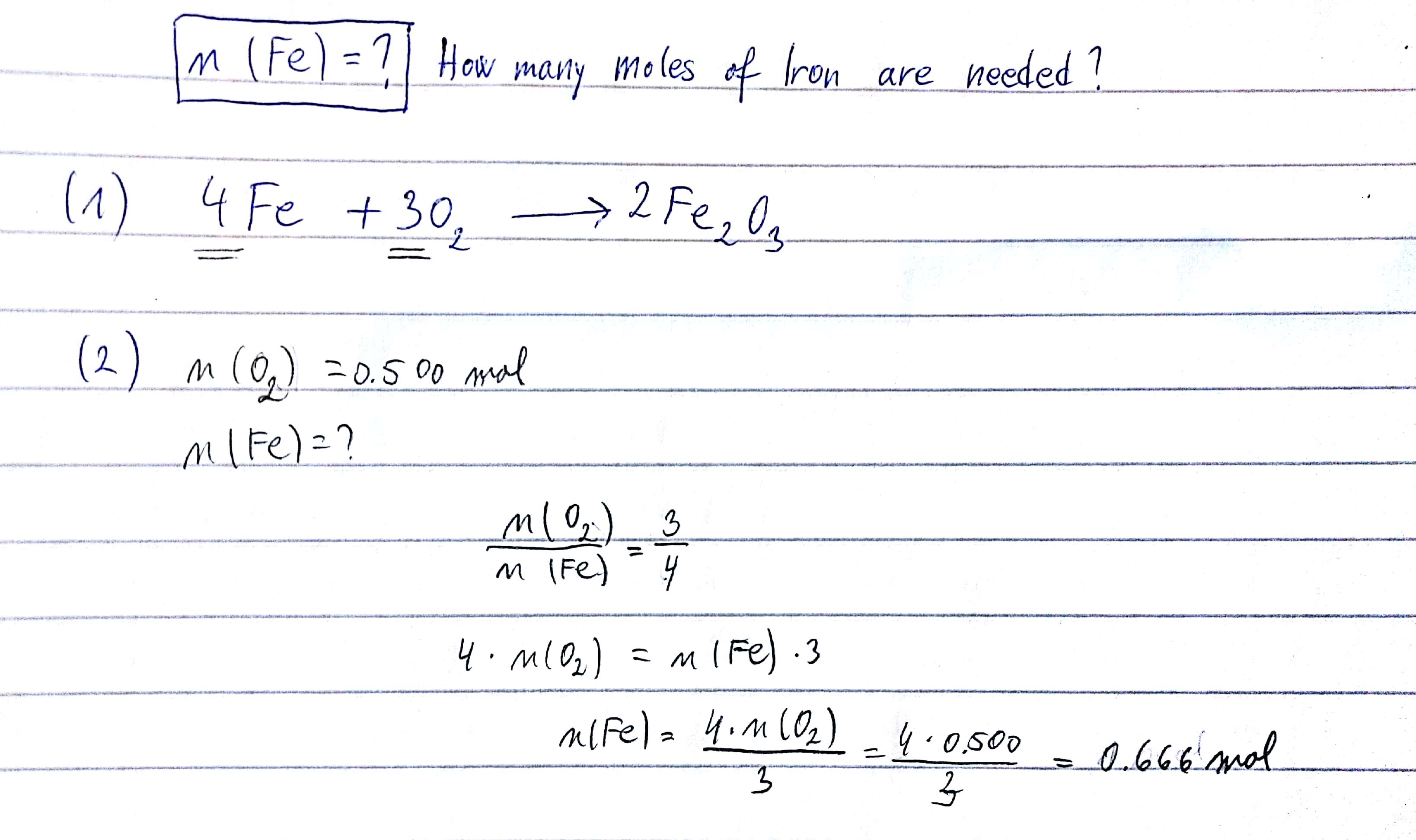How do you balance the equation 4 Fe(s) + 3 O2(g) → 2 Fe2O3(s) ?
1 Answer
Feb 11, 2018
I got 0,666 mol
Explanation:
- write the equation of the reaction and balnce it
4#Fe# (s) + 3#O_2# (g)#-># 2#Fe_2O_3# (s)
If you have problems with balancing this chemical equation, here is the whole process explained:
- put iron and oxygen [the reactants] in ratio to extract the number of moles for oxygen
On the picture below you can see how to work it out.