How Can Bonding And Antibonding Electron Can Be Identified In 2p Orbital?
2 Answers
It's a matter of energy
Explanation:
If you have no problem accepting that, for an hydrogen atom, it is the same electron can occupy different orbitals, e.g., 1s, 2p, 4d etc, then you shouldn't have no problem accepting bonding and antibonding electron could be the same electron.
Orbitals in an hydrogen atom have different [quantized] energy levels, the sole electron in the hydrogen can visit any of these orbitals as long as the electron has enough of energy to reach those states.
Because boding is a lower energy than anti-bonding state (of p orbitals or whatever), so the same electron can end up in the anti-bonding state depending on whether it has enough of energy to do so. You break the bonding if you give the electron enough energy, otherwise, it stays in the bonding state.
It cannot be.
Antibonding and bonding electrons can only be assigned when we are talking about molecular orbitals, i.e. within actual molecules. Otherwise, they do not exist.
They are called "bonding" and "antibonding" based on the molecular orbital they belong to.
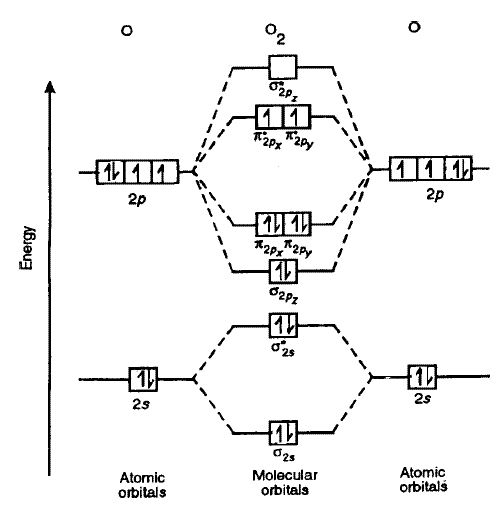
Consider the molecular orbital (MO) diagram of
 https://s3mn.mnimgs.com/
https://s3mn.mnimgs.com/
The bonding electrons that are in an MO constructed from the overlap of two
So,
- the
sigma_(2p_z) MO contains thesigma bonding electrons that were contributed as atomic electrons through the constructive head-on overlap of two2p_z atomic orbitals.
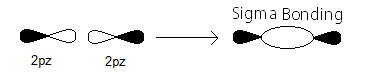
There are two such
sigma_(2p_z) bonding electrons.
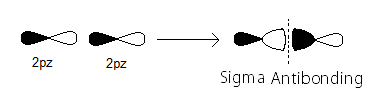
- the
sigma_(2p_z)^"*" MO contains thesigma antibonding electrons that were contributed atomic electrons through the destructive head-on overlap of two2p_z atomic orbitals.
There are zero such
sigma_(2p_z)^"*" bonding electrons.
Or, as another example,
- the
pi_(2p_x) MO contains thepi bonding electrons that were contributed as atomic electrons through the constructive sidelong overlap of two2p_x atomic orbitals.
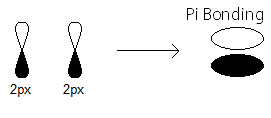
There are two such
pi_(2p_x) bonding electrons.
- the
pi_(2p_x)^"*" MO contains thepi antibonding electrons that were contributed as atomic electrons through the destructive sidelong overlap of two2p_x atomic orbitals.
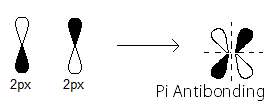
There is one such
pi_(2p_x)^"*" antibonding electron.