Question #2eafd
2 Answers
aluminium(Al)
Explanation:
this because aluminium has got three valence electrons in the outermost energy level hence has a stronger metallic bond/character than beryllium(Be) which has two valence electrons
Beryllium.
Explanation:
We can compare the metallic character of the two elements with reference to their electronegativity- both characteristics are related to an atom's ability to attract electrons in a compound.
Referring to the Pauling Scale of electronegativity values, we see that aluminum has an electronegativity value of
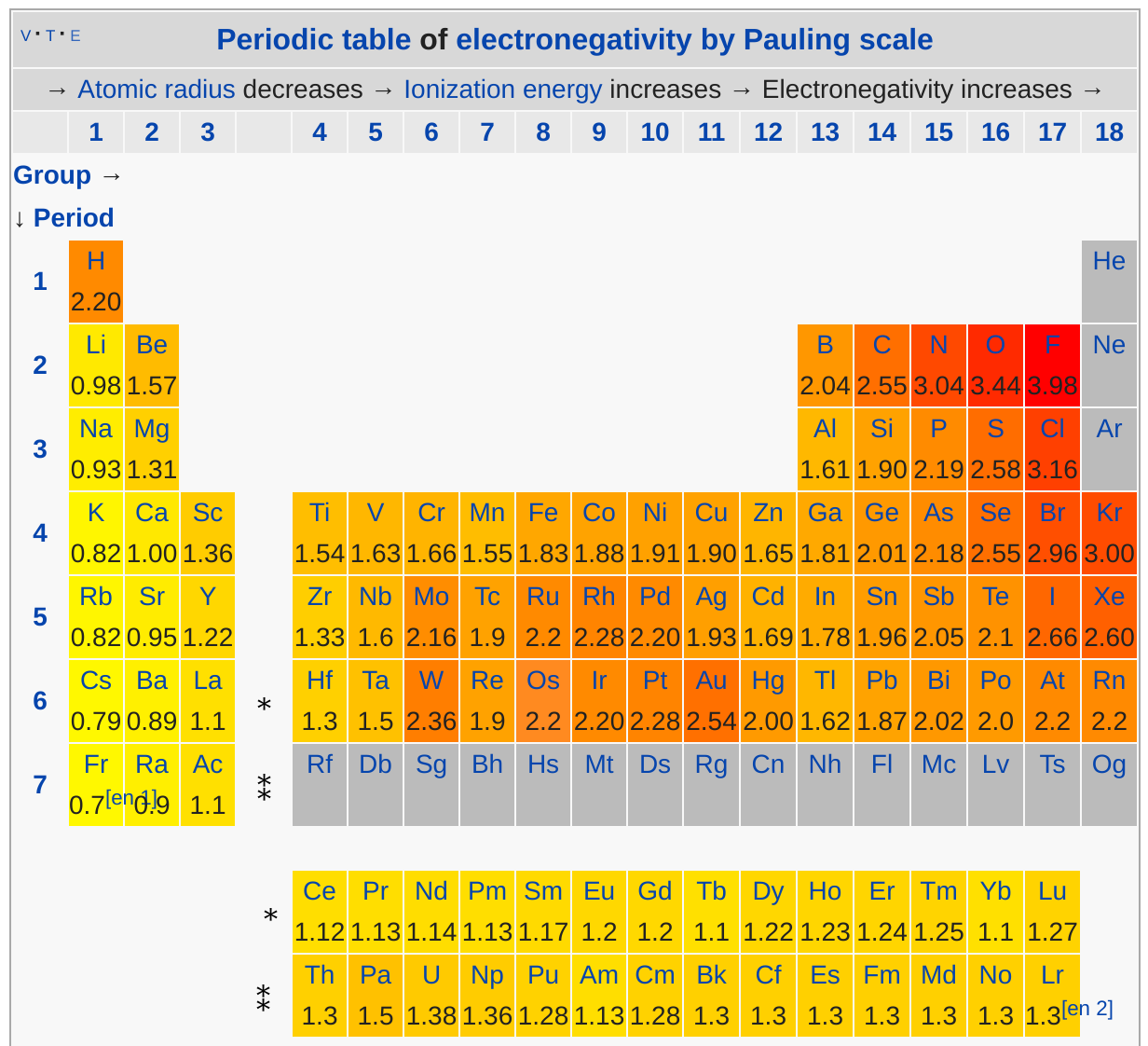
References:
Wikipedia contributors. "Electronegativities of the elements (data page)." Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, 25 Jan. 2018. Web. 1 Mar. 2018, https://en.wikipedia.org/wiki/Electronegativities_of_the_elements_(data_page)
Science, Ck12. “Periodic Trends: Metallic and Nonmetallic Character.” CK-12 Foundation, CK-12 Foundation, 30 Aug. 2016, www.ck12.org/c/chemistry/periodic-trends%3A-metallic-and-nonmetallic-character/lesson/Metallic-and-Non-metallic-Character-CHEM/.


