How you can explain that #C Cl_4# has no dipole moment?
2 Answers
Well, the molecular dipole moment....
Explanation:
....is the vector sum of the individual bond dipoles. There is some difference in negativity between carbon and chlorine, enuff to give a
On the other hand, chloroform,
See below...
Explanation:
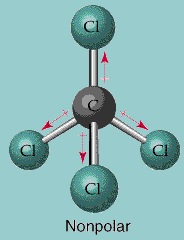
Let's have a vector approach.
- The angle between two
#"Cl"# atom is always#color(red)(109^@28'# .- So, we have to take the cos component of the three
#"Cl"# atoms showing in the pic downside.- I mark the upper one as
#(1)# , left one as#(2)# , bottom one is#(3)# and the right one is#(4)# .For
#(2),(3),(4)# no. chlorine,the resultant dipole moment is
#mu_((2-3-4))=3xxmu_1 cdot cos(180^@-109^@28')=3xxmu_1xx0.333=3xxmu_1xx1/3=mu_1" "# [#mu_1# is generated dipole moment for each#"Cl"# atom.]Now, the upper
#"Cl"# atom's dipole moment and the resultant dipole moment cancel out each other.Hence,
#color(red)(mu_("CCl"_4))=mu_1-mu_1=0#
This is the answer proved by vector.


