What does the strength of london dispersion forces between molecules depend on?
1 Answer
Mar 19, 2018
The number of electrons in the molecule....
Explanation:
...and therefore, to a first approx., to the atomic numbers of the constituent atoms in the molecule. And this is nicely indicated by the Noble Gas series, for which London forces are the ONLY intermolecular, here interatomic force...
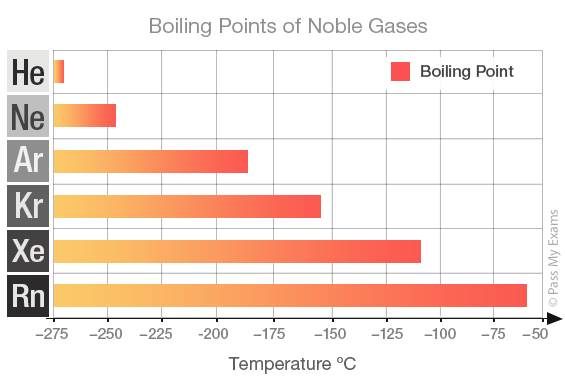
The bigger the electron cloud, the more polarizable it becomes, and thus many electron xenon and radon are the most involatile gases of those considered...

