Are dipole dipole forces stronger?
1 Answer
Depends on what they're being compared to.
Explanation:
Let's begin with a definition: Dipole-dipole forces are the intermolecular forces that are present in substances made up of polar molecules.
It results from when the slightly negative end of one polar molecule becomes attracted to the slightly positive end of another molecule:
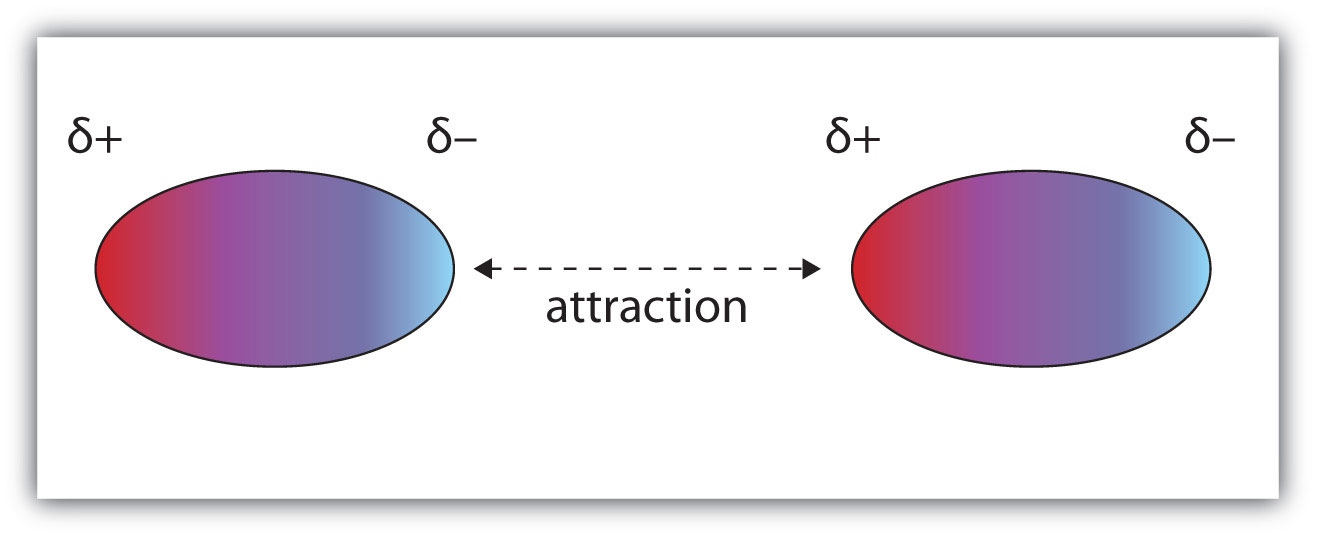
One type of dipole-dipole force that you might hear a lot is hydrogen bonding.
It's the strongest intermolecular force, and is only present in compounds with
So, if dipole-dipole forces are being compared to intermolecular forces like London dispersion forces, they would be stronger.
This is because London dispersion forces result from the attraction between non-permanent dipoles—see this answer by Owen Bell for a great explanation on them!
But if they're being compared to intermolecular forces like ion-dipole forces, which are forces that result from the attraction between a slightly charged dipole and a very charged ion (part of an ionic compound), they would be weaker.
This is because the attraction between two slightly charged poles is less than the attraction between one slightly charged pole and one very charged ion.
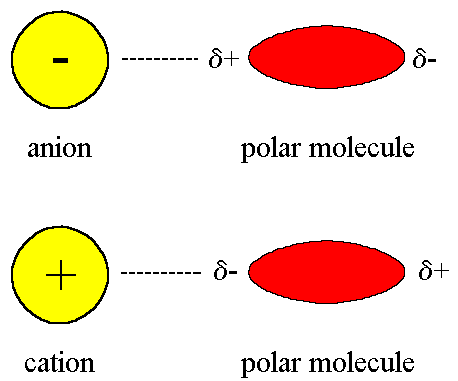
(The magnitude of ion-dipole forces will sometimes be greater than the ionic bond itself if there are a lot of polar molecules present, which is why polar molecules dissolve in water!)
If dipole-dipole forces are compared to intramolecular forces such as covalent bonds or ionic bonds, they would also be weaker.

