How does pressure affect phases of matter and intermolecular forces?
1 Answer
Atmospheric pressure will not affect intermolecular forces, but it will cause fusion and vaporisation, two phase changes, to require more energy.
Explanation:
(Assuming pressure refers to atmospheric, not vapor pressure.)
Some definitions first: Atmospheric pressure is the pressure of gas particles on the liquid or solid.
Vapor pressure is the pressure exerted by the gas particles of the liquid or solid (once the system is in dynamic equilibrium, which means that the number of gas particles leaving is the same number of gas particles that's coming back).
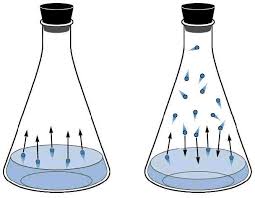
Vapor pressure is a thing because there will always be particles that can break free from the intermolecular forces of the liquid, causing it to become a gas.
Boiling point is when the vapor pressure of a liquid is the same as the atmospheric pressure:
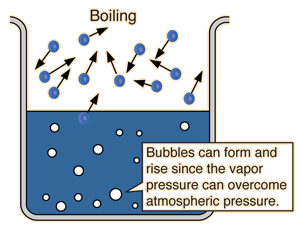
So, when the atmospheric pressure increases, the boiling point will also increase because it would be harder for vapor pressure to equal atmospheric pressure.
This is why vaporization, a change in phase, will require more energy.
However, the intermolecular forces in the liquid are not affected by this increase in atmospheric pressure.
Increasing the atmospheric pressure will generally increase the melting point of most substances, therefore increasing the energy required to facilitate fusion.
This is because of volume: Most solids have less volume than liquids, so melting would cause the solid to expand. Increasing pressure on the solid would make it harder for it to expand into a liquid.
Note how it says "generally," though! In some substances like water, the solid state has more volume than the liquid state due to hydrogen bonding, so increasing the external pressure will decrease the melting point of water.

