Why is benzene stable?
1 Answer
Benzene is stable due to the resonance that it shows.
Explanation:
The possibility of different pairing schemes of valence electrons of an atom is called Resonance and the different structures thus obtained are called resonance structures.
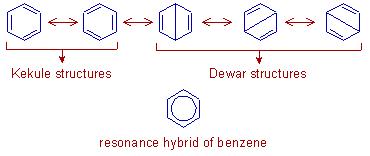
Benzene is actually collectively a resonance hybrid of all these five structures, consisting of three alternate single and double bonds which are known as the conjugate bonds.
Now what causes this resonance in benzene is the combination of six
Thus, the greater is this interaction, the more prominent is the resonance effect and hence the more stabler Benzene gets.
Infact, the more is the number of resonance structures possessed by a molecule, the more stable it becomes.
This is shown by the fact that despite being an unsaturated hydrocarbon(which give addition reactions), benzene gives substitution over addition reactions which would destroy the entire structure.

