How do exothermic reactions occur?
1 Answer
Explanation:
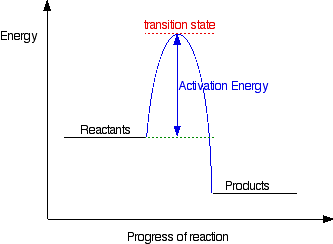
In any reaction, there is two stages. The Bond Breaking and Bond Forming stage which occurs after bond breaking.
Lets take
After that, free
Since in this reaction, the energy evolved(released) is larger than the energy absorbed to break the bonds, there is a net release of energy which results in the products having a lower energy content than the reactants as shown in the graph.
aka
