What causes bond polarity?
2 Answers
Unequal sharing of electron density in a covalent bond....
Explanation:
Take the
Electronegativity of atoms in the bond
Explanation:
Some atoms are more polar than others due to the fact that they are more "greedy" for electrons.
Take a look at your periodic table, the more right and upwards you go the more electronegative atoms become (not including the noble gases because they have full shells and are "happy" and don't need any valence electrons).
I think everyone has heard that Flourine (F), for example, is one of the most electronegative elements, it will literally rip electrons of Hydrogen atoms or carbon atoms. So will Chlorine (Cl)
Check out this image:
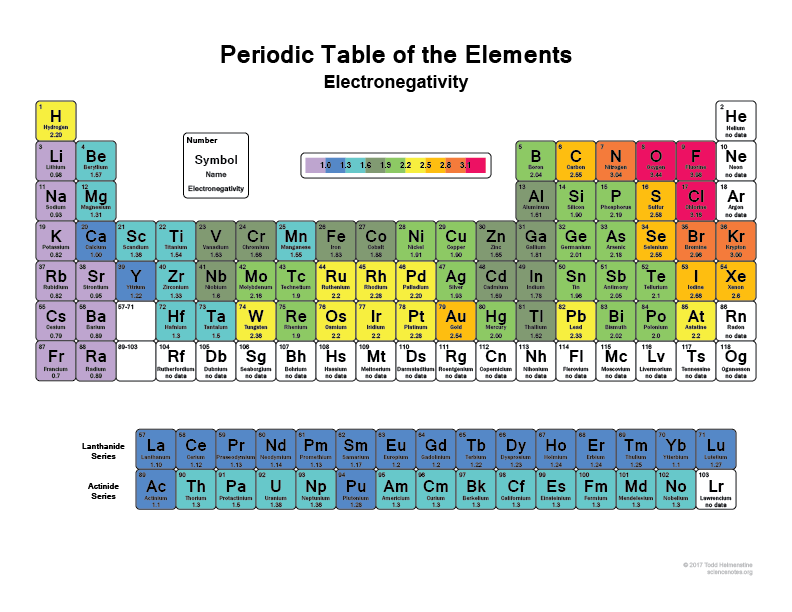
That's why when a bond some molecules have super polar regions because they have something like Hydrogen bonding with something like Flourine that just basically has the electrons with it most of the time.
This question can also be explained by the idea of electron clouds and the fact that more electronegative atoms will usually have more electrons staying with it most of the time versus not so much electronegative atoms.
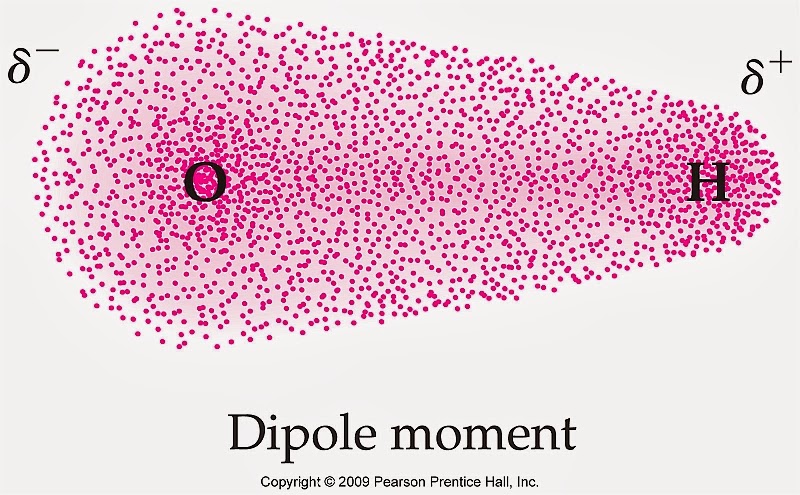
This is called the dipole moment and without getting too much into it you see how most the electrons are closer to the Oxygen than the Hydrogen (because Oxygen is more electronegative than Hydrogen)
By the way the word electronegative sounds counterintuitive sometimes but its electro-negative instead of positive because in chemistry atoms that gain electrons have a negative sign (Ex:



