Why does water expand when frozen?
1 Answer
May 12, 2018
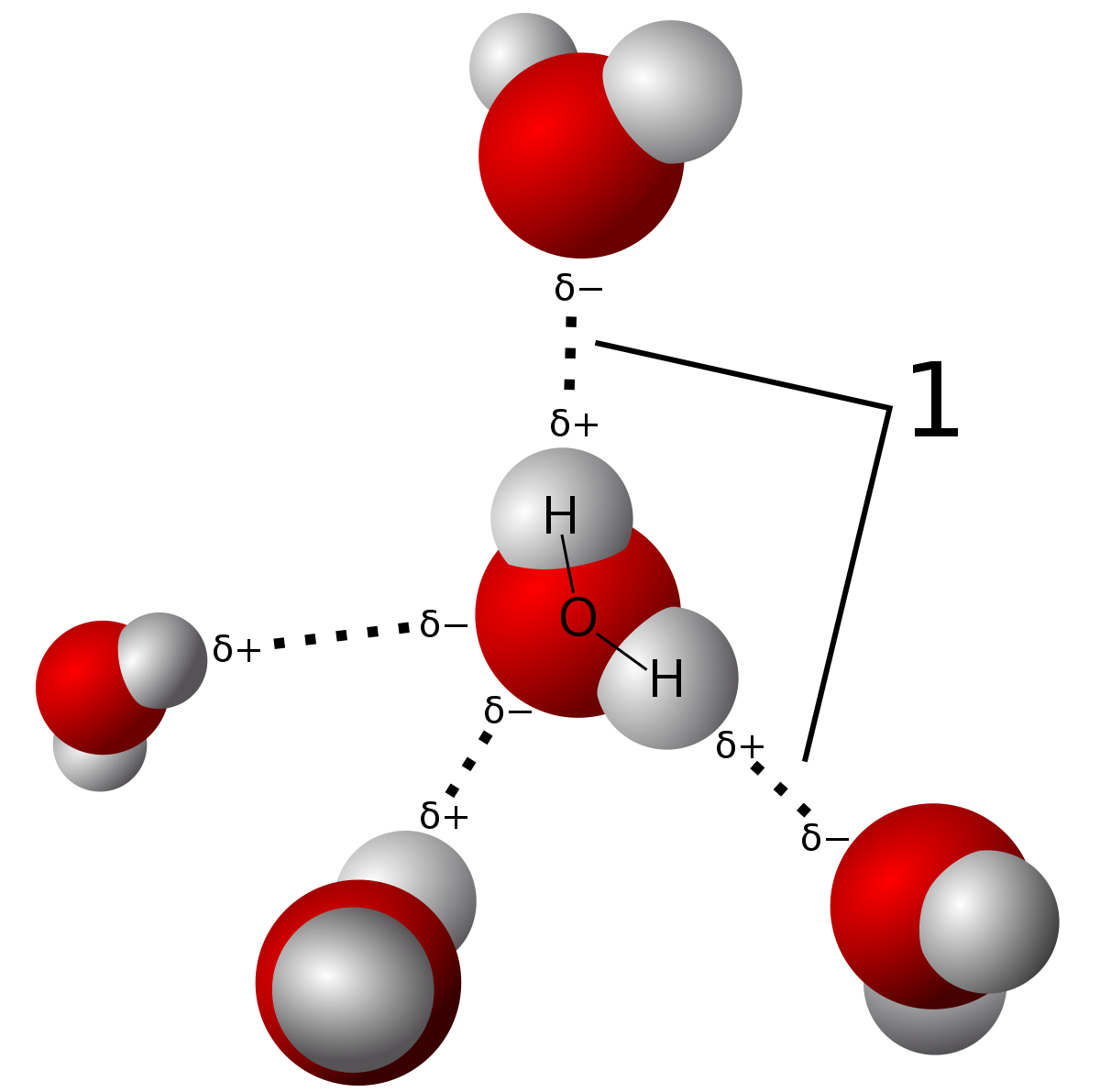
Due to hydrogen bonding...
Explanation:
Water is an unusual liquid, which has a lower density in its solid form than its liquid form, due to the extra volume in its ice form. This is due to a phenomenon called hydrogen bonding, and occurs when polar ends with opposite charges of water molecules get attracted to other water molecules. This is not a very strong bond though, and can be easily broken by heating up the solid a bit to over
In hydrogen bonding, there are a lot of empty spaces between the bonds, hence for the extra volume occupied by the ice.