What is the Lewis structure of #Cl_3#?
1 Answer
May 13, 2018
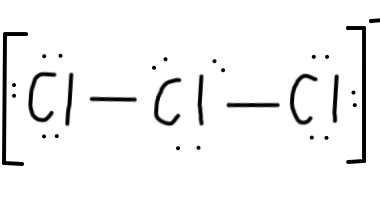
Explanation:
(Assuming that
- The total valence electrons for
#Cl_3^-# is#7xx3+1=22# , since#Cl# has#7# valence electrons and there's a negative charge (which adds#1# electron). - Let's draw a single bond between the
#3# #Cl# atoms to begin:
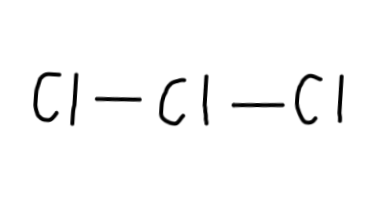
Now, we have#22-4=18# electrons left to put on the diagram, since each single bond counts as#2# electrons. - Then, we should complete the octets for all of the
#Cl# atoms:
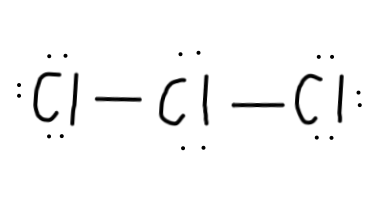
- We still have
#2# electrons left after placing all those valence electrons.#Cl# can have an expanded octet, though, so that's not too much of a problem. - To determine where to put this extra electron pair, let's calculate the formal charges of all the situations:
- If the extra electron pair is placed on the left
#Cl# atom, then the formal charge on that#Cl# atom would be#7-9=-2# . - If it were placed on the right
#Cl# atom, then that#Cl# atom would have a formal charge of#7-9=-2# . - If it were placed on the central
#Cl# atom, then that#Cl# atom would have a formal charge of#7-8=-1# . - So, the best placement for that extra electron pair would be the central
#Cl# , since that arrangement minimises formal charge. - Let's draw that:
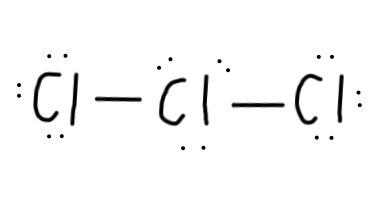
- And add a bracket around everything, with a negative sign, to indicate that this is a negatively charged ion:
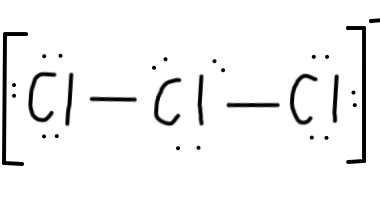
- Finally, let's do a last double-check for the number of electrons. We should end up with
#22# electrons. :)

