How does the size of metal atom and the oxidation state of the metal ion affect the CFSE ?
If the size of metal atom is small, it is more stable. So it should have higher CFSE. Similarly, higher charge on metal ion would give more stability and hence the CFSE should be more. Is this the correct relation?
If the size of metal atom is small, it is more stable. So it should have higher CFSE. Similarly, higher charge on metal ion would give more stability and hence the CFSE should be more. Is this the correct relation?
1 Answer
You may have the correct conclusion, but you'll want to re-examine your rationale. The whole point of crystal field theory is metal-ligand repulsions, not the stability of the isolated metal atom.
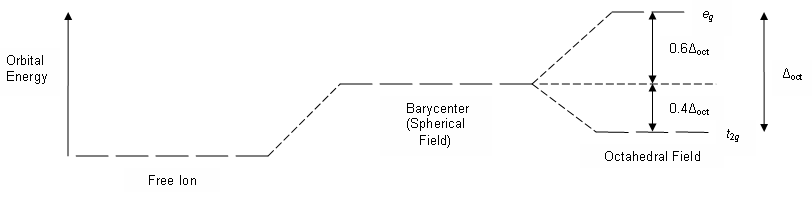
The crystal field splitting energy according to crystal field theory is based on the amount of metal-ligand repulsions, i.e. the repulsions between the most extended metal orbitals and the electron density of the ligand orbitals.
Consider an octahedral complex which has
 https://upload.wikimedia.org/
https://upload.wikimedia.org/
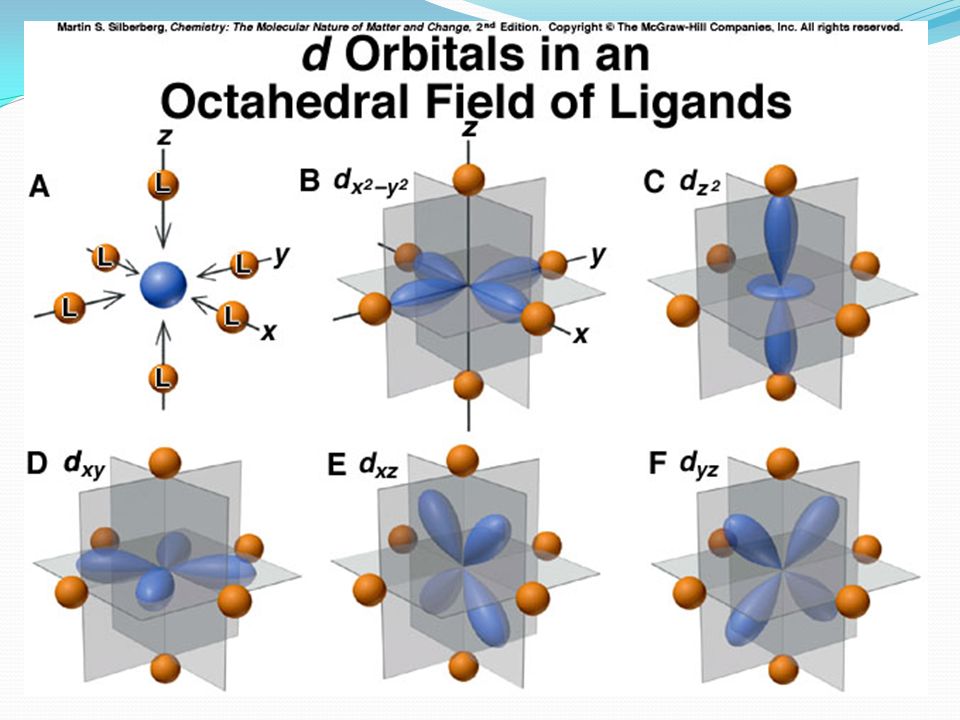
The interactions that arise are proposed to be driven by the attraction of the negatively-charged nonbonding ligand orbitals (modeled as point charges) to the positively-charged metal cation center.
This attraction leads to metal-ligand repulsions.
 Martin S. Sieberberg, Chemistry: The Molecular Nature of Matter and Change, 2nd ed.
Martin S. Sieberberg, Chemistry: The Molecular Nature of Matter and Change, 2nd ed.
- The more positively-charged the metal center, the more the ligands are attracted and thus the more repulsions the ligand orbitals have with the metal orbitals.
Therefore, we expect
Delta_o to be larger as metal oxidation state increases (for fixed metal size).
- Larger metals are larger because they have larger orbitals, which interact more (in obviously a repulsive manner) with incoming ligand orbitals.
Therefore, larger metal centers (for a fixed oxidation state) also increase
Delta_o .

