Why might a galvanic cell go dead?
1 Answer
The main reason is that the anode gets "used up," or depletes.
Explanation:
This is a galvanic cell:
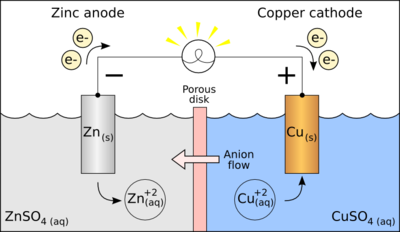
The two main components of a galvanic cell is the anode, which sends electrons, and the cathode, which receives these electrons.
In order for the galvanic cell to do work/not be dead, this flow of electrons through the wire, from the anode to the cathode, needs to be maintained.
In our diagram, the zinc metal is the anode. Copper is the cathode.
When this galvanic cell is discharging, the electrons flow from zinc to copper. As zinc metal loses electrons,
Our galvanic cell basically goes dead when all of the

