What are concentration cells?
1 Answer
A type of galvanic cell where the two half-cells are identical except for in concentration.
Explanation:
This is a concentration cell:
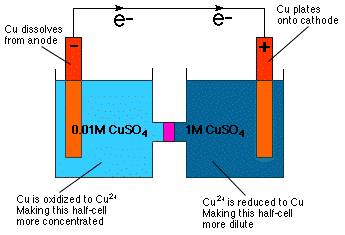
As we can see, the two half-cells are completely identical—the two electrodes are both made of solid
The only thing that's different in these half-cells is concentration of the
This will cause electrons to migrate in the direction that results in the increase in concentration of the
So, in our case, electrons will flow from left to right.
In the left half-cell, this causes
In the right half-cell,

