Why when the shape of molecules become more compact it's boiling point decrease while when intermolecular force become strong boiling point increase?
1 Answer
Well, they're two separate trends. The former is based on variation in London dispersion forces, and the latter is based on variation in intermolecular force types.
If the chosen intermolecular force is the same between both bases of comparison, then these trends are certainly consistent with each other.
COMPACTNESS OF THE MOLECULE
A more compact electron cloud has smaller volume, which typically means it has smaller contact surface area (provided the two systems are comparable in composition).
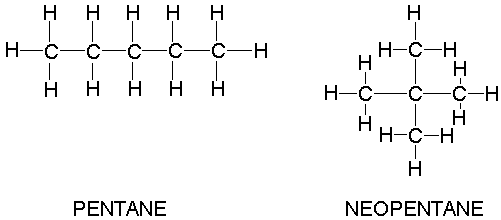
For example, take n-pentane (pentane) and neopentane, both
Because neopentane is more compact than n-pentane, but the chemical formulas are the same, neopentane has less contact surface area.
#"Less contact surface area on neopentane"#
#-> "Less London dispersion attractive interactions"#
#-> "The molecules can move apart more easily"#
#-> "Boiling is easier"#
#-> "Boiling point is lower for neopentane"#
In fact, the boiling point of neopentane is
INTERMOLECULAR FORCE STRENGTH
When intermolecular force strength increases, i.e. as we examine the following list from left to right
#"London dispersion" -> "dipole-dipole" -> "H-'bonding'" -> "ion-dipole" -> "ion pairing"#
we have that systems with predominantly the stronger intermolecular forces in this list, in terms of magnitude, TYPICALLY have higher boiling points.
[An exception is
The logic is similar.
#"Stronger intermolecular force of attraction"#
#-> "The molecules can move apart less easily"#
#-> "Boiling is harder"#
#-> "Boiling point is higher"#
Hence,
Examining the top three curves only, the far-left molecules have hydrogen-'bonding' forces of attraction, and the less-far-left molecules have dipole-dipole interactions as their respective predominant intermolecular forces.
Again,
#"Stronger intermolecular forces" -> "higher boiling point"#
Same with melting points.
#"Stronger intermolecular forces" -> "higher melting point"#